A Survey of Cryptosporidium Oocysts in Water Supplies during a 10-Year Period (2000-2009) in Seoul
Article information
Abstract
This study has been conducted to estimate the occurrence of Cryptosporidium oocysts in water supplies in the Metropolitan area of Seoul, South Korea, for 10 years from 2000 to 2009. Water samples were collected quarterly at 6 intakes in the Han River and its largest stream and 6 conventional Water Treatment Plants (WTPs) serving drinking water for 10 million people of Seoul. Cryptosporidium oocysts were found in 22.5% of intake water samples and arithmetic mean was 0.65 oocysts/10 L (range 0-22 oocysts/10 L). Although the annual mean of oocyst number was as low as 0.04-1.90 oocysts/10 L, 3 peaks in 2004 and 2007 were observed and the pollution level was a little higher in winter. The lowest density was observed at Paldang intake and the pollution level increased at Kuui and Jayang intakes. At the end of the largest stream, oocysts were found in 70% of collected samples (mean 5.71 oocysts/10 L) and it seemed that its joining the Han River resulted in the increase at Kuui intake and downstream. Oocyst removal by physical process exceeded 2.0-2.3 log and then all finished water samples collected at 6 WTPs were negative for Cryptosporidium in each 100 L sample for 10 years. These results suggested that domestic wastewater from the urban region could be a source of Cryptosporidium pollution and separating sewage systems adjacent to the intakes could be meaningful for some intakes having weakness related to parasitological water quality.
INTRODUCTION
Cryptosporidium is a small protozoan parasite that infects the microvillous region of epithelial cells in the digestive and respiratory tract of vertebrates. Infection in immunocompetent human hosts is predominantly caused by Cryptosporidium hominis, and the cattle genotype of Cryptosporidium parvum has also been reported to infect humans [1]. However, other Cryptosporidium species have also been reported to infect humans; less frequently (Cryptosporidium meleagridis, Cryptosporidium felis, and Cryptosporidium canis) or very occasionally (Cryptosporidium muris, Cryptosporidium andersoni, and the pig genotype of Cryptosporidium). Robust oocysts are shed by infected hosts into the environment and these oocysts can survive in the environment, such as water and soil, for months without losing their infectivity until ingested by a new suitable host. Transmission occurs through direct or indirect contact with feces of infected hosts and there can be various routes, such as person-to-person spread, animal contact, the digestion of polluted food or water.
These protozoan parasites have been recognized as a frequent cause of waterborne disease [2,3] because of their strong resistance against chlorine disinfection [4] and a low minimum infectious dose of oocysts [5]. These oocysts have been reported to be present in worldwide water environments [6-8]. In South Korea, there have recently been several reports on the cases of cryptosporidiosis [9,10] in humans and animals, although there have been no documented public outbreaks associated with drinking water. Therefore, evaluating their pollution levels in the source water, their removal efficiencies by conventional water treatments, and establishing the management strategy based on the results are urgently required.
In South Korea, a few studies [11,12] have reported the occurrence of Cryptosporidium oocysts in surface water environment but there have been no studies based on a long-term survey. Thus, the present study was conducted to determine the occurrence of Cryptosporidium in the Han River, the source water of municipal drinking water in the Metropolitan Seoul area over a 10-year period and to evaluate the removal efficiency of oocysts performed by the 6 conventional Water Treatment Plants (WTPs).
MATERIALS AND METHODS
The Han River as source of drinking water
The Han River is the only major water supply source for 20 million people of the Seoul Metropolitan region, which consists of Paldang Reservoir, an artificial lake that was created by a dam, and the downriver from the dam. From 2000 to 2009, surface water samples were collected at 6 sites along the river that included an intake at the end of the reservoir and 5 intakes on the downriver (they range over 25.2 km), quarterly (Fig. 1). These 6 intakes supply source water for 6 WTPs of Seoul. We also collected water samples at the end of Wangsuk stream, coming to the middle of the Han River, from 2000 to 2006. This stream has been strongly influenced by sewage treatment plants (STPs), treated sum of 0.16 × 106 m3/day by activated sludge treatment and the secondary effluents were discharged without any disinfection until 2004.
Water purification plants
We collected finished water samples quarterly from 6 WTPs of Seoul for 10 years, to evaluate the microbiological safety of drinking water. These plants serve approximately 10 million consumers of Seoul Metropolitan city by producing total 3.27-4.34 × 106 m3/day and use the Han River as their source water. At all plants, clarification process includes poly-aluminium chloride (PACl) coagulation, flocculation, and sedimentation. The clarified water passes through a single-media rapid sand filter, in which the depth and effective size of sand are mostly 1,150 mm and 0.9-1.12 mm. The filtered water is subsequently disinfected using chlorine gas and its normal residual free chlorine was 0.6-1.0 mg/L. During the winter when first 1-year survey had showed that the level of the parasites in raw water was the highest, settled and sand-filtered water was sampled at some plants with source and finished water, to evaluate the removal efficiency of Cryptosporidium oocysts by each treatment process of full-scale plants. The removal efficiency was calculated as follows:
Log removal = Log {(conc. at the inlet of the process)/(conc. at the outlet of the process)}
Isolation of oocysts from environmental water samples
Cryptosporidium oocysts were detected using USEPA Method 1623 [13], which combined concentration and immunomagnetic separation (IMS) with immunofluorescence assay (IFA). First, to concentrate very large volumes of water samples, capsule filtration technique (Envirochek™, Pall Corporation, Ann Arbor, MI, USA) was used for river, stream, and recycled water of 10 L. We also used disk membrane filtration (142 mm, 2 µm, Millipore Corporation, Bedford, MI, USA) or capsule filtration technique (Envirochek™ HV, Pall Corporation, Ann Arbor, MI, USA) for finished water samples of 100 L, Filta-Max™ filtration (IDEXX, Laboratories, Inc., Westbrook, Maine, USA) for more larger volumes of samples, and direct centrifugation for sewage effluents of 1 L. Materials on the filter were eluted and the eluate was centrifuged to pellet the oocysts, and the supernatant fluid was aspirated. The oocysts were purified by immunomagnetic separation (IMS) using magnetic beads conjugated to anti-Cryptosporidium antibodies (Dynabead GC-Combo, Dynal., Oslo, Norway).
Detection of Cryptosporidium oocysts
The purified oocysts were stained on well slides with fluorescence-labeled monoclonal antibodies (MeriFluor, Meridian Diagnostics, Cincinnati, Ohio, USA) and 4',6-diamidino-2-phenylindole (DAPI). The stained sample was examined using fluorescence and differential interference contrast (DIC) microscopy. Qualitative analysis was performed by scanning each slide well for objects that meet the size, shape, and fluorescence characteristics of Cryptosporidium oocysts. Quantitative analysis was performed by counting the total number of objects on the slide confirmed as oocysts. Objects with the typical fluorescence, size, and shape were enumerated as total Cryptosporidium oocysts, and subsequently confirmed as oocysts through nuclei staining or characteristics of internal structures.
Quality control of parasite detection
For quality control of this detection method, the recoveries from spiked control samples were analyzed routinely, using enumerated spiking suspensions prepared by flow cytometer (BTF EasySeed™, Sydney, Australia). Although detection limits varied with each type of sample because of the difference of filtered volume, the difference of recovery between each technique was not significant statistically. Routine matrix spike recoveries from river water samples for this study was 42% on average for oocysts (15-72%, relative SD = 37%), and when samples were concentrated by other techniques recoveries were similar.
Other water quality indicators
Various indicators, such as turbidity, particle counts, total nitrogen, total coliforms, aerobic spore formers, pH, and water temperature were tested. Because of the large numbers of non-detect measurements of Cryptosporidium in water samples, median or geometric mean was not appropriate to distinguish differences between sampling sites [14], and then in this study arithmetic mean was shown mainly with percent of positive samples. Microsoft Excel and Minitab program were used for statistical analysis.
RESULTS
Cryptosporidium in river water
Table 1 summarized the routine occurrence of oocysts in river water from 2000 to 2009. Confirmed oocysts with internal structures (1-4 sporozoites) (Fig. 2) or amorphous structure were found in 19.2% of intake water samples from the Han River and the arithmetic mean was 0.52 oocysts/10 L (0-22 oocysts/10 L). When the counts of empty oocysts (Fig. 3) were added, the total oocyst detection rate was 22.5% of intake water samples and the mean number was 0.65 oocysts/10 L (range 0-22 oocysts/10 L). These results were similar to earlier findings in the Information Collection Rule Supplement Survey by USEPA [14]. USEPA analyzing the source water of 80 WPTs in USA by USEPA Method 1623 reported that the mean density of oocysts was 0.06 oocysts/L.

Occurrence of Cryptosporidium oocysts in the river water samples selected periodically from 6 intakes of the Han River in South Korea (2000-2009, n = 240)
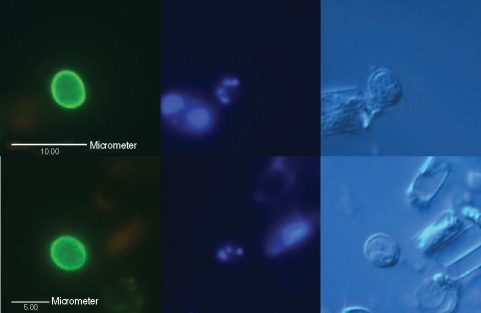
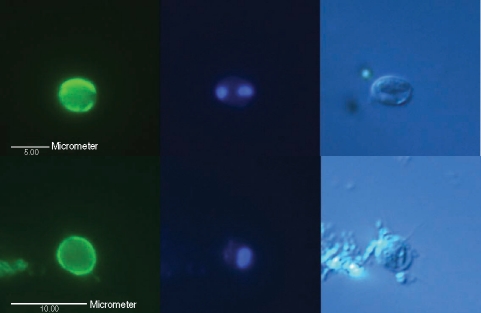
Micrographs of typical Cryptosporidium oocysts confirmed by fluorescence microscopy using differential interference contrast optics, found in 2 intake water samples (up and down) from the Han River. (Left) An oocyst showing its brilliant apple-green fluorescence with ovoid shape, stained with mAb, 5 µm in diameter under a blue filter; (Middle) 1-4 blue points by DAPI nuclei staining under a UV filter; (Right) 1-4 sporozoites shown by DIC.

Micrographs of an empty Cryptosporidium oocyst or oocyst-like object observed by fluorescence microscopy using DIC found in an intake water sample of the Han River. (Left) blue filter; (Middle) UV filter; (Right) DIC.
In environmental samples there were many oocyst-like objects that had similar apple-green fluorescence but showed atypical structures, such as 1 or 2 large nuclei filling the cell, red fluorescing chloroplasts, crystal, etc. by DIC or atypical DAPI staining (Fig. 4). Because of this, nuclei staining by DAPI and examination of internal structures by DIC should be critical for valid identification of oocysts in environmental water samples [15].
Difference of the oocyst pollution level at 6 intakes
The lowest concentration was observed in Paldang intake water samples (R1) with 0.20 oocysts/10 L as the arithmetic mean and 12.5% as the positive rate. Oocysts in Kangbuk (R2), Amsa (R3), and Pungnap (R6) intakes water samples were found in similar levels to R1 (0.28 to 0.40 oocysts/10 L) (Table 1). Each maximum value at 4 intakes was in the range of 3-5 oocysts/10 L. But the pollution level of oocysts was higher at Kuui (R4) and Jayang (R5) intakes, in which the mean number of total oocysts was 1.73 oocysts/10 L and 0.90 oocysts/10 L, respectively. Samples with more than 6 oocysts/10 L were found 3 times at R4 and once at R5. As a result of analysis of variance, oocyst concentration in R1, R2, R3, and R6 intakes had statistically significant difference from that of R4 and R5 intakes (P < 0.05). It means that there could be the inflow of the polluted water between R2 site and R4 site.
Cryptosporidium in the largest stream, Wangsuk
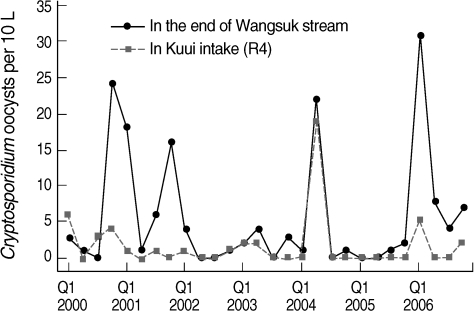
At the end of Wangsuk stream, oocysts were found in 70% of samples and the mean number was 5.71 oocysts/10 L (0-31 oocysts/10 L, n = 28). Wangsuk stream is the largest tributary coming to the middle of the Han River, in which treated effluents from 2 STPs make up about a half of its water. Lee et al. [12], who surveyed the oocyst pollution level of at Wangsuk stream and these 2 STPs in 1999, reported that oocysts were positive in 32% of 1-L discharge samples of these STPs and the mean in the discharge and Wangsuk stream water samples was 0.5 oocysts/L and 2.0 oocysts/10 L, respectively. Although we did not investigate on STPs in this study, it seemed that the pollution level of oocysts at Wangsuk stream was still similar to or little higher than in 1999, even after the operation of ozone disinfection process at STPs since 2005.
Also the occurrence of oocysts at R4 intake joining the stream was significantly correlated with those at Wangsuk stream (r = 0.552, P = 0.002) and most of peaks at Wangsuk stream resulted in the increase at R4 intake (Fig. 5). This suggests that domestic wastewater from the urban region was a source of Cryptosporidium pollution in the river and separating sewage system adjacent to intakes can be meaningful in the Metropolitan area of South Korea. But similarly low density of oocysts in 6 intakes in ordinary condition (Table 1) may reflect that the source of Cryptosporidium is from the upper reaches of Paldang reservoir, especially livestock wastewater from the rural region.
Finished water at 6 water treatment plants
In quarterly finished water samples (n = 240) collected at 6 conventional WTPs during 10 years, no Cryptosporidium oocysts were detected in each sample of 100 L. Total coliforms were also not detected in all 100 mL samples. It is suggested that the oocyst number might be routinely lower than 10 oocysts/100 L, which is the action level to prevent an outbreak [6,16]. It means that Seoul WTPs in the Metropolitan area of South Korea were estimated to remove oocysts effectively to prevent outbreak under the present level of pollution of source water.
DISCUSSION
During the 10 years, the annual average number of oocysts in the river water samples was as low as 0.04-1.90 oocysts/10 L (Fig. 6), which did not show any significant difference between STPs, even though 2 STPs have operated ozone disinfection process since 2005. It showed that ozone disinfection at STPs may be insufficient for inactivation of Cryptosporidium oocysts. There were 3 peaks in May 2004 and March 2007 observed at R4 and R5 sites and it means that these intakes were weaker in microbiological water quality control.
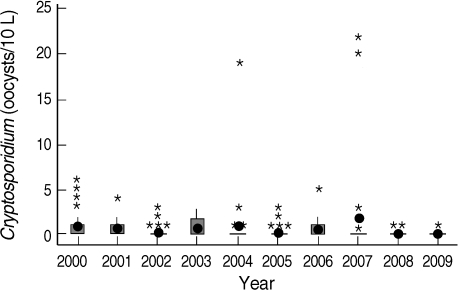
Yearly distribution of Cryptosporidium oocysts in the Han River water samples (2000-2009). • mean, * higher value than the mean, □ Range between the first quartile and the third quartile.
Generally oocysts in river water were more frequently found in winter. The positive rate of 60% for oocysts on the first quartile was higher than 7.5-13.8% of the second, third, and fourth quartiles. The mean density of oocysts was 1.6 oocysts/10 L in winter and 0.25-0.45 oocysts/10 L in other seasons. This seasonal trend was found repeatedly. It seems to be related to the stronger resistance of oocysts in low temperature and the lowest rainfall during winter in South Korea. Although Yu et al. [10] reported that the prevalence of cryptosporidiosis among the villagers in some rural areas of South Korea was the highest in June and Chai et al. [17] reported that seasonal peaks of cryptosporidiosis appeared in spring and autumn, the time interval between shedding of oocysts and detection in water environment may be long, and environmental survival of oocysts can be influenced by various factors, such as the distance and routes from the source to the environment, temperature, and dilution effects [18].
Pearson correlation coefficients (r) between oocysts and other water quality parameters were computed. As a result, all surface water samples with Wangsuk samples showed the correlation of some water quality parameters. Despite too many non-detection data, the occurrence of total oocysts in surface water showed the correlation with total nitrogen (r = 0.463, P < 0.0001), NH3-N (r = 0.335, P < 0.0001), total coliforms (r = 0.300, P < 0.0001), fecal coliforms (r = 0.285, P < 0.0001). Although previous studies suggested that coliforms were not a helpful indicator for parasite pollution [19,20], it seems that coliforms could be used as an indicator in case that surface water was under the direct influence of sewage. No confirmed oocysts were detected in surface water samples with less than 50 CFU/100 mL of total coliforms, although confirmed oocysts were not always detected in samples with excess 50 CFU/100 mL of total coliforms. However, none of the correlation coefficients was statistically significant with regard to turbidity and pH.
Log removal of Cryptosporidium and various indicators at 4 WTPs was summarized in Table 2. As Cryptosporidium oocysts are strictly resistant against chlorine disinfection, physical removal processes, such as sedimentation and sand filtration are very important and critical. But it was very difficult to determine the log removal efficiency at the full-scale WTPs due to very low levels in the source water. As a result, we could determine only that Cryptosporidium removal by physical process exceeded 2.0-2.3 log, because no oocysts were detected in all sand-filtered water samples. These results were comparable with some previous studies reported 2-3 log removal on full-scale plants [21-23], but showed lower removal than on the previous pilot studies spiked with high concentration of oocysts [24,25].

Range (mean) of log removal of Cryptosporidium and various indicators by sedimentation and filtration at conventional water treatments in South Korea
Log removal of oocysts and that of 3 indicators, such as turbidity, particle counts, and aerobic spore formers, did not show statistically significant correlations. The lack of correlation may be partially because log removals of oocysts were influenced by their densities in the source water. However, the turbidity of sand-filtered water samples was less than 0.15 NTU (mean 0.10 NTU, range 0.07-0.15 NTU) and average turbidity of settled water samples was 0.68 NTU (range 0.52-0.78 NTU). The results show that physical processes at studied plants were operated under optimal conditions during this study and conventional treatments could remove above 2.0 log of Cryptosporidium, if operated properly.
ACKNOWLEDGEMENTS
The authors thank Office of Waterworks, Seoul Metropolitan Government for long-term supports and Prof. Jong-Yil Chai, Department of Parasitology and Tropical Medicine, Seoul National University College of Medicine, for careful and helpful advices on this study.