A Survey of Dung Beetles Infected with Larval Nematodes with Particular Note on Copris lunaris Beetles as a Vector for Gongylonema sp. in Iran
Article information
Abstract
Dung beetles (family Scarabaeidae) are one of the largest families of beetles worldwide. Due to biological behavior of these arthropods, they are considered to play an important role in the life cycle of some helminths. In the present study, dung beetles collected from cattle pastures in rural areas of Ardabil province, north-west of Iran were examined for infection with larval stages of helminths. According to the results, nematodes of 2 genera were identified including Rhabditis and Gongylonema. The more common species was Rhabditis sp. which was found in 9 species of beetles. Out of 15 different species of dung beetles, Copris lunaris was the only scarabaeid to be found naturally infected with the larval stages of Gongylonema sp. Our new findings introduce C. lunaris as a potential biological vector for transmission of Gongylonema sp. to vertebrates in the surveyed region.
INTRODUCTION
The role of dung beetles as hosts of helminths [1] has brought them to the attention of parasitologists. Scarabaeidae (Coleoptera) and Diptera are among those well recognized representatives of the coprophagous community, as their biological behavior in decaying dung pats is obviously highlighted [2-4]. There are several studies demonstrating that dung beetles are abundantly attracted to human stools, quickly bury their bodies, and consume large quantities of feces [5]. Meanwhile, animal dung, especially cattle droppings, has long been regarded to serve as food and microhabitat for both adult and larval stages of beetles [6]. This biological behavior will bring dung beetles in close contact with parasites eggs in human and animal excretions on the ground.
Gongylonema spp. (Molin, 1857) (Nematoda: Spiruroidea) are nematode parasites belonging to the superfamily Spiruroidea, with about 25 species in many wild and domestic mammals and 10 species in birds, worldwide [7]. This nematode has an indirect life cycle, and about 50 species of arthropods such as beetles and cockroaches are possible intermediate hosts [8]. Adult worms harbor in tunnels into the squamous epithelia of the upper digestive tract, mainly throat and esophagus of the definitive hosts, where eventually eggs are laid and pass out with the feces into the environment [9,10].
Transmission of infection to both humans and animals is basically similar, which could result from an accidental or intentional ingestion of food items contaminated with whole or parts of infected arthropods by humans or through grazing in pasture surfaces by ruminants. Human gongylonemiasis is widely distributed, although it appears rarely in review of literatures. The species Gongylonema pulchrum has been reported in humans as sporadic cases in many places around the world, including 10 times in the United States [11].
In Iran, the first case of human infection with Gongylonema has recently been reported in a 35-year-old woman with a 1-year history of feeling migratory creeping sensation in the neck region and the upper part of the digestive tract [12]. Prevalence of infection among ruminants in the country has indicated as 45% in cattle [13] and 21% in sheep [14]. However, relatively little attention has been paid for identification of larval stages of this nematode among invertebrate hosts in the nature. Consequent to the first documentation of human gongylonemiasis in the country and repeated records on the existence of Gongylonema spp. in ruminants, attention has been directed in the present study to look for its invertebrate vector in areas with a history of animal infections.
MATERIALS AND METHODS
This study was carried out in Ardabil province (38°15'N, 48°17'E) in north-westernn Iran. This province is about 70 km far from the Caspian Sea and 25 km from the Republic of Azerbaijan's border. It has an average altitude of 1,263 m and total area of 18.011 km2. Its annual maximum and minimum temperaturas are 35℃ and -25℃, respectively.
In summer of 2005, a total of 231 dung beetles were collected manually from cattle pastures, mostly from fecal deposits and the adjacent surface soil, in rural areas of 5 different towns including Namin, Neer, Meshkinshahr, Sarein, and Ardabil city. The specimens were transferred to our laboratory in plastic holders. At the time of examination most of them were still alive, therefore aiming to make them immobile and ready for manipulation, beetles were placed into a refrigerator for a few hours. In the first step, species identification of scarabaeids was carried out taxonomically, using available keys [15,16]. Afterwards, all beetles were dissected in normal saline solution and examined carefully with a stereomicroscope for the presence of nematode larvae. In case of infectivity, larvae were removed and placed into normal saline, kept for 1 hr on the icy surface to become relaxed. Leaving the larvae into lactophenol for a few hours provided them enough cuticular transparency, helpful to achieve desirable results for further identification steps. Morphological characteristics for every specimen were then accurately drowned by camera lucida, at × 400 magnification and careful identification was comparatively pursued based on key references [17,18].
RESULTS
Overall, among 231 individuals of dung beetles collected from rural areas of 5 districts in Ardabil province, 15 species belonging to 9 genera were identified. Among those, Onthophagus taurus was the most abundant species (34.19%), while Onthophagus amyntas, Onthophagus speculifer, and Onthophagus furcatus abundancy were the least (each with 0.86% rate). From the geographical distribution points of view, Meshkinshahr and Sarein showed the most and the least frequency of dung beetles, i.e. 45.0% and 3.9%, respectively (Table 1).
In general, 2 genera of nematodes were identified from all collected dung beetles (Fig. 1). Multiple infestations were not found in any of the examined beetles. The common nematode was Rhabditis sp. (Fig. 2) which was found in 8 species of beetles, in 3 regions, constituting 17.7% (41 of 231) infection among all collected dung beetles. The beetle species harboring Rhabditis are introduced in a colored map (Fig. 1). The other nematode with peculiar importance was identified as Gongylonema sp. (Fig. 3) exclusively in Copris lunaris beetles with a total infection rate of 2.2% (5 out of 231 total collected beetles). C. lunaris was present in Ardabil city and in 2 other regions including Namin and Meshkinshahr, but the Gongylonema-infected beetles were only observed in Namin (Fig. 1) where 5 out of 13 captured individuals (65%) were found infected. The larval burden for each infected beetle was 1, 2, 2, 3, and 6.
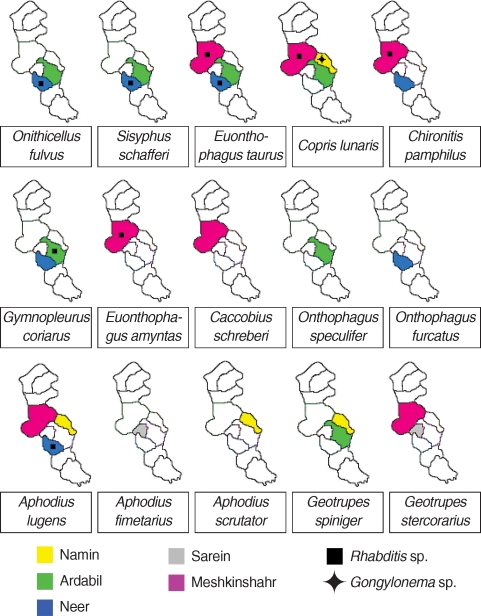
Map of Ardabil province, Iran, representing the distribution of various species of dung beetles in different areas and their status of infection with Rhabditis sp. and Gongylonema pulchrum larvae.

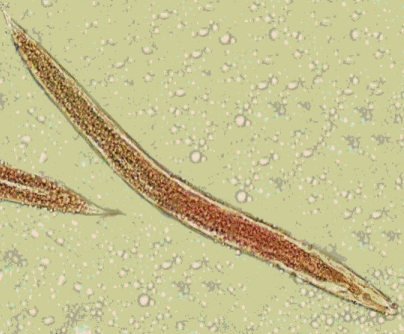
(A) A larva of Gongylonema pulchrum recovered from a Copris lunaris beetle (× 100). (B) Anterior part of a larva showing the mouth part at a higher power view (× 400).
Identification of the third larval stage of Gongylonema sp. was justified by referring to characteristics described by one of the rare reliable references throughout the literatures [17,18]. The anterior end of the larvae showing the typical mouth parts (Fig. 3B) with average measures including the total length of 2.15 mm, width at mid-body 0.08 mm, length of the muscular esophagus 0.65 mm, length of the glandular esophagus 0.76 mm, and distance from the anus to the posterior end of the body 0.1 mm. These measurements were comparatively agreed with what had been documented so far for G. pulchrum 3rd stage larvae. For Rhabditis larvae, diagnosis was carried out based on morphological features completed with the average measures of a total length of 925 µm, distance between mouth and the end of esophagus of 58 µm provided by the same reference as well as Yamaguti's collections [18].
DISCUSSION
Concerning the potential role of dung beetles in transmittion of certain helminths such as acanthocephalans and spirurid nematodes to humans and animals, the faunistic study of the parasites they harbor seems to be of great veterinary and medical importance. The coprophagous fauna such as Scarabaeidae (Coleoptera) are the most important agents promoting dung pat decay [12-14].
G. pulchrum is a spirurid nematode found in many species of mammals such as cattle, sheep, goats, camels, pigs, equines, cervids, rodents, bears, and primates worldwide [7]. Although it is primarily a parasite of ruminants, there are several available records of human infections with Gongylonema in the world [11, 12]. Its route of transmission to animals seems to be through usual grazing; however, in humans the factors that may put some people at increased risk of infection with this spirurid parasite are not clearly understood. Observation of the first human case of gongylonemiasis in Iran [12] in addition to many records of infection in livestocks [13,14] promoted us to search for invertebrate intermediate hosts in this region.
Based on personal observations of gongylonemiasis among slaughtered animals, Ardabil province was selected for the present survey. Our new findings proved the existence of 15 species of dung beetles that would be likely much more diverse in the area if the field of study, as well as seasonal sampling, had been considered more widely. This study showed a 19.9% (46 of 231) infection rate of all examined beetles with 2 kinds of nematode larvae. Introducing C. lunaris for the first time as a potential vector of Gongylonema in Iran, which has not been stated in the world publication before, could be considered as the main remarkable finding of this survey. Meanwhile, C. lunaris as the only beetle species found to be carrying Gongylonema larvae among 15 dung beetle species, beside the finding of Seuart in 1916 based on the role of C. hispana as an intermediate host for Spirocerca lupi, is indicating that the genus Copris would be likely to be one of the most susceptible arthropods to harbor larval stages of spirurid nematodes [19].
The other kind of nematode detected in this study was Rhabditis sp. This nematode exists abundantly in different kinds of soil worldwide [20]. Despite several reports of human infections with free-living nematodes [21], they are not well considered as the real threatening agents of human and animal health. In the present study, 41 out of 231 (17.7%) collected dung beetles were found carrying these free-living nematodes internally, as well as on external body surfaces. Seven species of beetles collected in this survey including Caccobius schreberi, O. speculifer, O. furcatus, Aphodius fimetarius, Aphodius scrutator, Geotrupes spiniger, and Geotrupes stercorarius did not show any infection with Rhabditis sp. This finding is of great importance from the perspective of eco-biology. Meanwhile, the collected scarabaeids from Namin and Sarein areas were found free of any soil living nematodes. Capability of certain species of beetles for harboring Rhabditis worms along with the geographical determinant factors is among the interesting subjects of debate.
Comparing the prevalence of Gongylonema sp. and Rhabditis sp., 2.2% and 17.7%, respectively, could be strongly attributed to the complexity of Gongylonema life cycle compared with that of free-living nematodes which can easily be obtained from the soil everywhere. The exclusive existence of Gongylonema parasites among C. lunaris beetles of Namin area seems to be related with poor hygienic condition of this location and the larger sample size allocated for Namin.
In conclusion, this survey has proved the presence of infected intermediate hosts of Gongylonema, presumably G. pulchrum, in northwest of Iran. Therefore, with regard to observation of adult worms in local slaughtered animals, control measures must be adequately considered to prevent humans from infection with this rare but a potential agent of so-called delusional parasitosis because of its migrating behavior around the oral cavity and throat in human patients [12]. Of course the appropriate procedures of control in health sectors will definitely improve the general status of environmental hygiene which subsequently would be protective against soil-transmitted nematodes as well. Concerning the prevention measures in veterinary sectors, i.e., proper overall management, improving environmental hygiene, providing clean feed and water supplies, and aplication of suitable pesticides, if necessary, will all be helpful to minimize the chance of dung beetle infection with this nematode.
ACKNOWLEDGEMENTS
We would like to thank colleagues Mrs. Farzaneh Zahabioon for her kind collaboration, Mr. Ali Rahimi and Miss Neda Mirsepahi for providing help during the survey.