Antibody Responses in Sera of Different Mouse Strains Experimentally Infected with Neodiplostomum seoulense
Article information
Abstract
To examine humoral immune responses in the host, we measured serum antibody levels in different strains of mice (ICR, BALB/c, and C3H) experimentally infected with Neodiplostomum seoulense. Specific IgG antibody levels were increased remarkably with little difference among 3 strains of mice infected with N. seoulense from day 7 to 35 post-infection. More target proteins of adult parasites reacted with IgG at the time when the worm recovery decreased compared with other times. More than 20 protein bands, from 14 kDa to 94 kDa in size, were separated from the crude antigen of N. seoulense adults by SDS-PAGE, and among them 26, 30, 35, 43, 54, 67, and 94 kDa proteins were the major antigenic proteins. The results suggest that significant IgG antibody responses occur against N. seoulense in mice and this may be related with expulsion of worms.
Neodiplostomum seoulense (Digenea: Neodiplostomidae) is an intestinal fluke of humans and rodents in the Republic of Korea [1,2]. The first record of its human infection was a young man who complained of severe gastrointestinal troubles, fever, and eosinophilia after eating raw or undercooked snakes [3-5]. Subsequently, 25 additional human cases have since been reported [4,5]. The first intermediate host of N. seoulense is the freshwater snail Hippeutis cantori, and the second host is the tadpole and frog of Rana nigromaculata, whereas the grass snake Rhabdophis tigrina plays the role of a paratenic host [1].
In experimental mice and rats, the main habitat of N. seoulense is the duodenum, but parasite locations extend to the jejunum and ileum in heavy infections [6]. This fluke has been shown to be highly pathogenic and lethal to mice within 1 month after experimental infection [7,8]. Severe degenerative changes of villi, hyperplasia of crypts, mastocytosis, and goblet cell hyperplasia have been observed in the small intestines [9,10]. Mucosal mast cells and goblet cells were markedly increased; however, proliferation of these cells was considered merely a result of local immune responses due to the presence of worms, rather than playing important roles in the host defense and worm expulsion [8,10].
In one of the well-known intestinal parasite, Echinostoma caproni, the parasite is known to evoke significant humoral immune responses in mice. Antibodies, such as IgM, IgG, and IgA, have been detected in the serum and the small intestine of infected mice [11,12]. Similarly, specific serum and mucosal tissue IgA increased in mice which had been experimentally infected with N. seoulense, although the increase was not directly related to the expulsion of the worm [13]. Thus, the humoral immune responses related to worm expulsion and host damage remain to be elucidated.
In the present study, serum levels of Neodiplostomum specific IgG were measured by ELISA in 3 strains of mice (ICR, BALB/c, and C3H) experimentally infected with N. seoulense. Furthermore, target proteins of the parasite specifically reacting against the IgG antibodies in the serum were analyzed by SDS-PAGE and immunoblot.
BALB/c mice raised under specific pathogen-free (SPF) conditions were purchased from an animal breeding company (Dae Han Biolink Co., Eumsung, Chungbuk, Republic of Korea). Mice were 7-wk-old males at the beginning of the experiment, and were maintained at a SPF animal facility. European grass snakes, Rhabdophis tigrina, naturally infected with N. seoulense were purchased from Hongcheon, Gangwon-do, Republic of Korea. Metacercariae were isolated using an artificial gastric juice containing 0.5% pepsin (1: 10,000) (Sigma Chemical Co., St. Louis, Missouri, USA) and 0.8% HCl, as previously described [14]. Metacercariae were used to infect rats and mice and to prepare the metacercarial antigen. Three strains of mice (ICR, BALB/c, and C3H) were used for infection with N. seoulense. Mice were orally inoculated with 200 metacercariae in 0.2 ml of saline using a gavage needle. Then, 5-6 mice from experimental and control groups were killed every 7 days, and examined for immune responses. Blood was collected, and sera were separated and stored at -70℃ until use.
Immunosuppressed rats were infected with N. seoulense metacercariae and killed with an overdose of ether at day 14 post-infection (PI). Adult worms were recovered from the small intestines, and washed with saline. The soluble antigen mixture of adult worms was prepared by homogenizing worms in phosphate-buffered saline (PBS) using a glass-teflon homogenizer. After centrifugation at 10,000 rpm, supernatants were collected and stored at -80℃ until required. Soluble antigen concentrations were determined using the Lowry's method [15].
Indirect ELISA was established for detecting specific IgG antibodies in the sera of N. seoulense-infected mice. In brief, the crude worm extract was diluted to a concentration of 10 µg/ml in carbonate buffer (pH 9.6), and coated on 96-well microtiter plates. The mouse sera were diluted to 1 : 100 in PBS-0.05% Tween 20 (pH 7.4) and dispensed to the plates which were incubated for 2 hr at 37℃, followed by adding horseradish peroxidase-conjugated goat anti-mouse IgG (Caltag Lab., Burlingame, California, USA) diluted in 1 : 1,000. Color was developed by adding 0.01% o-phenylenediamine in 0.03% H2O2 in phosphate-citrate buffer (pH 5.0) and incubating for 30 min. Absorbances were measured at 492 nm using a microplate reader (Molecular Device EMax, Sunnyvale, California, USA), and the specific absorbance values of test samples were determined after subtracting the absorbance of serum-free wells.
The crude antigen of N. seoulense was separated using 7.5-15% polyacrylamide gradient gels under reducing conditions. The proteins resolved by SDS-PAGE were transferred onto a PVDF membrane, and reacted with the sera of N. seoulense-infected mice and negative controls in 1 : 100 dilution, followed by soaking with horseradish peroxidase-conjugated goat anti-mouse-IgG diluted in 1 : 1,000. The final reactions were developed with 4-chloro-1-naphtol and H2O2.
The worm recoveries and optical densities of IgG for each group of mice were represented as their arithmetic means and standard deviations (SD). The statistical significance among the groups was tested by the Student's t-test. The values of P < 0.05 were considered significant.
On day 7 PI, the average worm recovery rates (WRRs) from ICR, BALB/c, and C3H mice were 39.9%, 29.5%, and 16.2%, respectively (Fig. 1). The WRRs in ICR and BALB/c mice were significantly higher (P < 0.05) than those in C3H mice. After day 7 PI, the WRRs began to decrease in all mouse strains. On day 14 PI, the average WRRs from the 3 strains of mice were 38.8%, 25.5%, and 14.2%, respectively. The WRRs decreased to 29.4%, 24.8%, and 9.8% on day 21 PI, and further decreased to 26.2%, 20.1%, and 6.5%, respectively, on day 28 PI. The WRRs decreased visibly to 11.9% in ICR mice, and 17.0% in BALB/c mice on day 35 PI. However, all mice died in C3H mice before day 35 PI. Regarding ICR mice, the WRRs decreased until days 28 and 35 PI, which were significantly lower (P < 0.05) than the value on day 7 PI. However, there were no significant differences from day 7 PI to days 28 and 35 PI for the WRRs between BALB/c and C3H mice (P > 0.05).
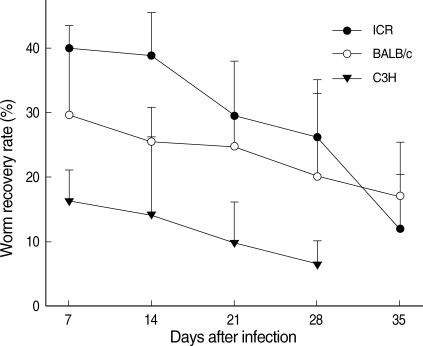
Comparison of the chronological worm recovery rates (%) from ICR, BALB/c, and C3H mice infected with 200 metacercariae of Neodiplostomum seoulense.
The IgG antibodies against N. seoulense adults were detected in the sera of all infected mice on days 7, 14, 21, 28, and 35 PI (Fig. 2). The absorbance values of IgG exhibited unique patterns through the course of infection in each mouse strain, ICR (Fig. 2A), BALB/c (Fig. 2B), and C3H (Fig. 2C). All 3 strains of mice developed intense responses of specific IgG antibodies against the N. seoulense adults (Fig. 2). The antibody titers increased progressively over the course of infection to reach the maximum value on day 28 PI in 3 strains of mice, and thereafter the values steadily declined until day 35 PI in ICR and BALB/c mice. Statistically significant differences (P < 0.05), compared with uninfected controls, were observed in ICR mice from day 14 PI and in BALB/c mice from day 7 PI until the end of the experiment. The similar results were shown in C3H mice from day 21 PI through day 28 PI.
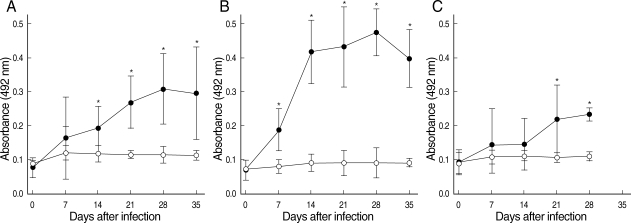
Comparison of absorbances at 492 nm for IgG antibodies in ICR (A), BALB/c (B), and C3H mice (C) infected with Neodiplostomum seoulense. Infection sera were tested against the crude antigen of N. seoulense adults. Expressed as the mean absorbance values of control (○) and infected mice (•) over the course of infection. Vertical bars represent the standard deviation. Asterisks represent significant differences in comparison with uninfected controls (P < 0.05).
More than 20 protein bands, from 14 kDa to 94 kDa in size, were separated from the crude antigen of N. seoulense adults by SDS-PAGE. Among these protein bands, 26, 30, 35, 43, 54, 67, and 94 kDa proteins were major proteins (Fig. 3A). On immunoblot, 40, 54, 70-90, and 96 kDa bands of the crude antigen reacted with the sera from ICR, BALB/c, and partly C3H mice. The 96 kDa and 70-90 kDa proteins were the common and the earliest occurring bands among the 3 strains of mice on day 7 PI (Fig. 3B-D). In immunoblot with sera from ICR mice, 30, 37, 43, 50, 60, and 100 kDa bands were observed from day 14 PI. On day 35 PI, most of the ICR mouse sera were reacted with 30, 37, 43, 50, 60, 67, 70-90, and 100 kDa antigens. With regard to BALB/c mice, the sera were reacted with 35, 37, 40, 54, 69, 70-90, and 96 kDa from day 14 PI. In C3H mice, 70-90 kDa and 96 kDa bands were observed from day 14 PI, and additionally 40 kDa and 52 kDa bands from day 21 PI.

Immunoblot patterns of the crude antigen (A) of Neodiplostomum seoulense with sera of ICR (B), BALB/c (C), and C3H mice (D) infected with N. seoulense at day 7 to 35 PI. Con: negative controls.
The results obtained were helpful to gain further insight into the host-parasite relationships in N. seoulense infection. We studied the serum antibody kinetics and antigenic protein profiles in 3 strains of mice to examine the potential role of antibody responses in host-protective immunity in N. seoulense infections. All 3 strains of mice developed intense serological responses in the total specific IgG against N. seoulense crude antigen. The kinetics of the total specific IgG in the serum of mice was consistent with earlier studies on E. caproni infections [16-18]. Serum IgG responses were remarkably greater in mice than in rats probably in relation to a greater intestinal antigen uptake and higher local inflammation [19]. In E. caproni-infected mice, increases in both IgG1 and IgG2 subclasses were observed, which could reflect a balanced Th1/Th2 local response and worm expulsion appears to be related to an increase in local IgG2a levels [18]. Although IgG subclasses were not examined in our study, it is suggested that at least IgG1 and/or IgG2 may be involved in the high levels of the total serum IgG. Although little is known about the mechanisms operating in the natural definitive host of N. seoulense, cellular mechanisms could be of great importance in determining the course of infection.
Circulating and secretory IgA responses have been related to expulsion of intestinal trematodes and protozoans [20,21]. However, previous studies suggested that increases of IgA were not sufficient for worm rejection of N. seoulense and E. caproni [13,18]. In N. seoulsense infection, specific serum IgA antibodies were shown to increase from day 7 to 28 PI, and local IgA reactions were observed in the mucosa and submucosa of the duodenum, though not directly related with worm expulsion. The infection in mice may be able to stimulate high and rapid IgA antibody responses at both systemic and local levels. The high levels of IgA could explain consequences of a dominance of Th2 responses observed in sera of mice.
A comparative study of N. seoulense infection in BALB/c and C3H reported that the WRRs from BALB/c mice were consistently and remarkably higher than those from C3H mice [10,14]. The extent of mucosal mastocytosis, goblet cell hyperplasia, and mucin activation was remarkable in the duodenum of BALB/c mice than C3H mice. These previous results have suggested that intestinal mastocytosis and goblet cell hyperplasia are local immune responses, and they may play minor roles in the host defense and worm expulsion [10,14].
In the present study, all 3 strains of mice presented the highest WRRs at day 7 PI, and subsequently exhibited marked capacities to expel primary infections of the parasite, and the WRRs decreased rapidly after day 7 PI. In this context, the analysis of IgG antibody responses in the host may be of considerable interest. We could find that specific IgG antibody responses against N. seoulense infection progressively increased and reached a maximum at day 28 PI in 3 strains of mice. This agrees to the previous studies on the antibody responses in mice infected with E. caproni [11,17,22]. From our results, IgG titers of both ICR and BALB/c mice were remarkably higher than in C3H mice, and the correlation between the IgG titers and worm expulsion was high and many antigenic band profiles were observed in western blot data. In C3H mice, however, the mucosal and humoral immune responses were not effective for the host defense, and a lethal pathogenicity was caused by irreversible damages to their intestines [7]. Thus, further studies are required to clarify the pathogenesis of N. seoulense infection.
A cysteine proteinase, having the molecular weight of 54 kDa, was purified from the crude extract of N. seoulense adults [23]. However, its precise role in eliciting pathogenicity to the host has not been elucidated. In the present study, a 54 kDa crude antigenic band was shown to react with mouse sera infected with N. seoulense from days 21 PI until the end of the experiment. This antigenic band might be an enzyme responsible for the host tissue lysis and penetration.