Sarcocystis and Its Complications in Camels (Camelus dromedarius) of Eastern Provinces of Iran
Article information
Abstract
The prevalence of Sarcocystis spp. was investigated by gross and histopathological examinations in 250 camels (Camelus dromedarius) slaughtered from 2002 to 2005 in the Mashhad Slaughterhouse, eastern Iran. Samples were taken from the diaphragm, heart, tongue, esophagus and masseter muscles for histopathological studies. No macroscopic sarcocysts were found in the samples at gross inspection. Sarcocysts were detected in 209 of 250 (83.6%) examined camels at histopathological level. The infection rate of the esophagus, heart, masseter muscles, diaphragm, and tongue was 58.8%, 48.0%, 46.8%, 41.6%, and 28.0%, respectively. There was no significant difference in the rate of infection between male (85.8%) and female (81.0%) camels. The tissue response to vital cysts was minimal; however, reaction to the degenerating cysts was severe and caused tissue damages resulting in hyperemia, hemorrhages, mononuclear cell infiltration, necrotic changes, and fibrosis. The wild and domestic carnivores especially dogs may be the final hosts of Sarcocystis spp. in this area.
INTRODUCTION
Sarcocystis spp. are common parasites with worldwide distribution in man and many species of animals, and infect the skeletal and cardiac muscles [1]. They are intracellular protozoan parasites of the phylum Apicomplexa. Camels are the intermediate hosts and become infected after ingestion of the sporulated oocysts passed in the feces of carnivores as the final hosts. The sporozoites excyst in the gut of the camel, undergo a period of rapid divisions in the gut wall, and eventually migrate to the striated muscles to form the characteristic sarcocysts. The life cycle is completed when the sarcocyst containing infective bradyzoites is ingested by the final host, which in the case of Sarcocystis cameli has been shown to be of the family Canidae [2-4]. After a period of multiplication involving asexual and sexual reproduction sporulated oocysts are passed in the feces of the final host [4].
The macroscopic species do not appear to be important pathogenic agents in this country or elsewhere [5]. The microscopic species that are not detected during routine meat inspection and are not responsible for carcass condemnation are the infective species of these zoonotic protozoa. Nevertheless, the high prevalence of the microscopic forms of Sarcocystis has a definitive economic impact on domestic animals production [5-7]. They can affect growth and weight gain of the animals, reduce the meat quality, and reduce milk yield, cause anorexia, fever, anemia, abortion, muscle weakness, and even death of the intermediate hosts [8-12].
Humans may acquire infection by consumption of undercooked meat of infected animals with certain species of Sarcocystis [4,13]. It is stated that human intestinal sarcocystosis is a zoonotic disease caused by 2 species of coccidian parasites, namely Sarcocystis fusiformis (syn. Sarcocystis bovihominis, S. hominis by consuming raw infected beef) and Sarcocystis meischeriana (syn. Sarcocystis suihominis by consuming raw infected pork) that can develop digestive disturbances, such as nausea, vomiting, stomachache, diarrhea, and dyspnea [4,14,15]. Man is the definitive host for S. hominis and S. suihominis with cattle and pigs as intermediate hosts for these species, respectively [16]. Humans also serve as the intermediate hosts for several unidentified species of Sarcocystis [17]. Based on the published reports, it appears that intestinal sarcocystosis is more common in Europe than in other continents [4]. Sarcocysts have been found in striated muscles of human beings, mostly as incidental findings [4]. Based on the published reports, human muscular sarcocystosis is a rare infection [4]. Beaver et al. [18] reviewed case histories and histological sections of reported cases of muscular sarcocystosis critically, and concluded that most reported cases were acquired in the Far East and Southeast Asia. There is no report so far on transmission of S. cameli to human beings owing to consumption of camel meat in Iran and other countries.
Mason [19] gave the first description of Sarcocystis infection in a camel (Camelus dromedarius) in Egypt. However, there are at least 2 morphologically different, thin and thick-walled, Sarcocystis affecting camels, though S. cameli is the only species reported from this animal [20-22]. This Sarcocystis species in camel is reported from Sudan, Jordan, Kazakhstan, Afghanistan, Morocco and former U.S.S.R [4,20], Egypt [23], Somalia [24], Saudi Arabia [21], Iraq [25], Southern Ethiopia [26], and Mongolia [27].
The Prevalence of Sarcocystis species in other slaughtered animals is also high in this country [7,28]. There are only 2 previous investigations on the prevalence of sarcocysts infection in camels from Tehran and Esfahan Provinces in the central area of Iran [29,30]. Rahbari et al. [29] examined the heart, esophagus, and diaphragm of 39 camels macroscopically and by impression smear method, and found a prevalence rate of 52.6%. Shekarforoush et al. [30] tested different skeletal muscles and heart of 400 camels by the same method and reported an infection rate of 52.3%.
Therefore, the present study was designed to determine the prevalence and tissue distribution patterns of Sarcocystis infection together with the histopathological changes caused by the parasites in camels of the eastern provinces of Iran.
MATERIALS AND METHODS
Numerous camels (C. dromedarius) are raised in the semi-arid regions in eastern part of Iran, mostly for meat consumption purposes. In some parts of Iran, people used to consume camel meat which has good quality and is economically fair in comparison to beef and sheep meat. This study was conducted on camels slaughtered at Mashhad Slaughterhouse, Khorasan razavi Province, eastern Iran. The camels brought to this slaughter-house from eastern provinces of Iran include Khorasan razavi and Sistan Bluchestan Provinces, that are arid and semi-arid areas of low annual rainfall.
A total of 250 camels, 134 males and 116 females, aged 1-16 yr, slaughtered during the period April 2002 to March 2005 were examined for Sarcocystis spp. Tissue samples from the tongue, heart, esophagus, diaphragm, and masseter muscles were considered for investigation. For macroscopic sarcocysts, all the skeletal muscles of the fore and hind limbs, diaphragm, intercostal muscles, heart, tongue, esophagus, and masseter muscles were carefully inspected by naked eye examination. For microscopic studies 3 pieces of each of the diaphragm, heart, tongue, esophagus, and masseter muscles were collected and fixed in 10% neutral buffered formalin. The samples were dehydrated in graded ethanol series, embedded in paraffin, and histological sections of 5 µm in thickness were prepared from 3 samples of each of the above organs, stained with hematoxylin and eosin, and examined under a light microscope. Data analysis was performed using the chi-squared test.
RESULTS
The naked eye examination did not reveal any macroscopic sarcocyst during the carcass examination, whereas, out of the 250 adult camels examined, 209 (83.6%) were positive for sarcocystosis by a histological examination. These positive results were from 115 out of 134 males (85.8%) and 94 out of 116 females (81.0%) for microscopic sarcocysts. There was no significant difference (P > 0.05) in the prevalence rate between the 2 sexes of camels. The prevalence of microscopic cysts in different tissues and sexes is shown in Table 1. The esophagus was the highest (58.8%), and the tongue was the lowest (28.0%) infected organ.
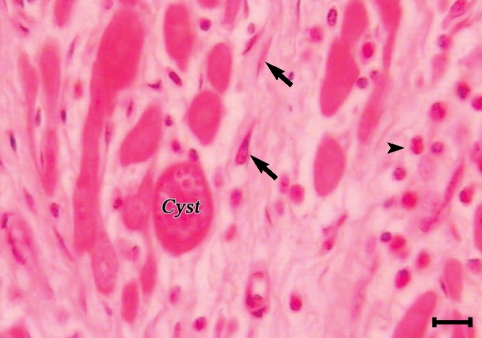
The sarcocysts of all the tested organs had similar morphologies and were of thin-walled type. Most of the affected tissues were free from any degenerative or inflammatory changes but in some of the infected tissues, the cysts were found in foci of tissue necrosis and appeared to be degenerated. While vital cysts initiated no inflammatory reactions (Figs. 1, 2), different phases of inflammation from acute stages including hyperemia, hemorrhages, and polymorphonuclear cell infiltration to chronic form reactions of various intensities were observed in response to the degenerating cysts. Macrophages, lymphocytes, plasma cells, eosinophils, fibroblasts, and connective tissues were infiltrated around these degenerated cysts. However, the tissue response was normally focal but in some instances especially in the cardiac musculature, lymphocytes, plasma cells, macrophages, eosinophils, fibroblasts, and fibrous connective tissue was diffusely infiltrated between muscle fibers (Figs. 3, 4). In some sections no inflammatory cell infiltration was seen but there was an increase in the interstitial connective tissue which spread the muscle fibers apart.
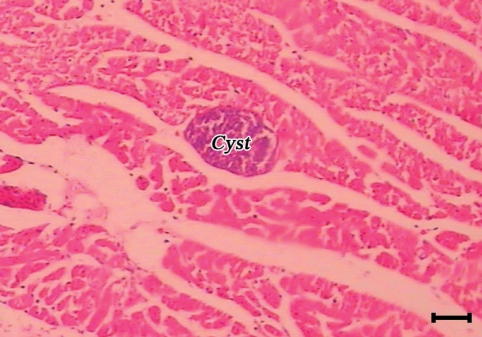
A sarcocyst (cyst) in the masseter muscle of a camel. The cyst initiated no tissue reaction (H-E stain, Scale bar = 75 µm).
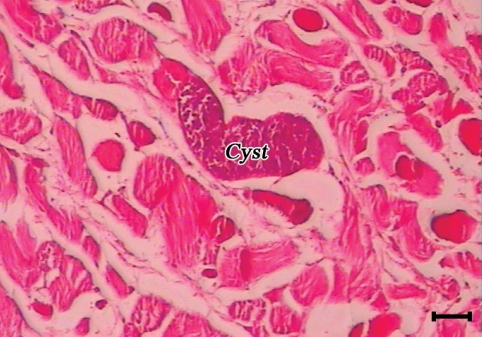
A sarcocyst (cyst) in the esophagus of a camel. The cyst initiated no tissue reaction (H-E stain, Scale bar = 75 µm).
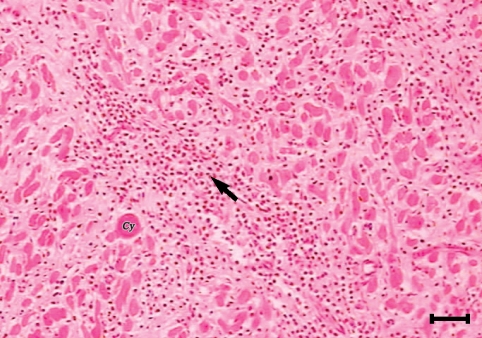
A sarcocysts (cy) in the myocardium of a camel. Eosinophils and mononuclear cells (arrow) are heavily infiltrated between muscle fibers. In some places fibroblasts and connective tissue substituted the cardiac muscle fibers (H-E stain, Scale bar = 180 µm).
DISCUSSION
The results of the present study revealed a high prevalence of Sarcocystis infection among camels slaughtered in the Mashhad Slaughterhouse. Whereas no macroscopic species were seen in different examined muscles, the prevalence of Sarcocystis was high (83.6%) at a histopathological level. The prevalence rate of Sarcocystis in camels in the present investigation is high, similar to our neighboring countries, including Saudi Arabia (88.4%) [21], Iraq (91.6%) [25], Mongolia (100%) [27], and Afghanistan (47.3-66.3%) [31]. Also reports from some African countries, such as Southern Ethiopia (45.5%) [26] and Sudan (81%) [32], show high incidence of Sarcocystis in camels.
The only 2 previous reports of Sarcocystis in camels in Iran by Rahbari et al. [29] and Shekarforoush et al. [30] showed lower infection rates than our findings. They reported infection rates of 52.3 and 52.6%, in Esfahan and Tehran Provinces, respectively. This big difference between the prevalence of Sarcocystis in camels in the present study and that of previous 2 studies in Iran may be firstly due to the differences in the system of camel maintenance by animal husbandry in central parts and eastern areas of Iran. Normally in the central areas such as Tehran and Esfahan the camels are maintained in close barns in a limited area, while those in the eastern provinces are kept by nomadic Assyrians and are grazed freely in different deserts. This high prevalence can be explained by the fact that the animals are raised by the nomadic Assyrians who keep large numbers of sentinel dogs with their camel herds. Moreover, wild carnivores such as hyenas, wolves, and jackals are common in nomadic areas and all of these are likely to contaminate pastures and especially the drinking water with Sarcocystis sporocysts.
In a previous study, the prevalence of Sarcocystis infection was significantly higher in animals owned by nomadic Assyrians than those owned by local people, because the former people were more exposed to this infection, owing to their close association with dogs and many wild carnivores that serve as the definitive hosts for this parasite [7]. Human feces are another source of pasture contamination in these areas. Human beings and primates appear to serve as intermediate hosts for several unidentified species of Sarcocystis [1,33]. It is not known if human beings become infected with uncooked or undercooked infected camel meat or not. Development of cystic forms of Sarcocystis in the striated and cardiac muscles of human beings after eating uncooked or undercooked meat of water buffalo and cattle is previously reported [33,34]. Moreover, it is possible that the camel Sarcocystis may have man as its final host, as in the case of S. hominis and S. suihominis, however, further investigations are needed to clarify this hypothesis. This is important since camel meat is consumed by people in this country and also many other countries in the world.
Secondly, the places of animal maintenances of each investigation were far from each other and with various geographical conditions and climate. Compared to the central provinces, the eastern provinces, Sistan Bluchestan and Khorasan razavi Provinces, are more arid with much lower annual rainfalls and as such with different population of animals as definitive hosts. The third reason could be due to the differences in the methodologies of the investigators. In the present experiment, 3 sections of each of the esophagus, tongue, heart, diaphragm, and masseter muscles of each animal were examined at a histopathological level, while other investigators applied impression smear method on fewer tissue organs with microscopic inspections.
The tissue distribution of Sarcocystis in different organs of camels reported by different investigators is also variable. While the results of the present study and those reported by Rahbari et al. [29], Shekarforoush et al. [30] and Woldemeskel and Gumi [26] showed that the esophagus was the most infected organ, Ibrahim [35] and Fatani et al. [21] reported that the overall infection rate was highest in the diaphragm, and Hussein [36] reported that the highly affected tissue was the tongue of camels. These differences may be due to differences among Sarcocystis strains of various definitive host origin and the methods applied in the sarcocyst detection. Two morphologically different thin and thick-walled Sarcocystis were previously reported in camels [4,21]. Dubey et al. [4] mentioned that the thick-walled cysts were found repeatedly and therefore they named it S. cameli and left the thin-walled cysts unnamed. In contrast to this finding, Fatani et al. [21] showed that the thin-walled cysts were more common. The difference in the rate of distribution of Sarcocystis in different organs of camels at various localities could be due to differences in the definitive hosts of these Sarcocystis species. Careful studies are needed on the camel Sarcocystis from Iran and other parts of the world to determine the most common types of Sarcocystis before the name S. cameli or other names can be given to any of these cysts. There are no reports about the molecular criteria and immunodiagnosis of different species of Sarcocystis in camels. These rare ultrastructural studies also concentrated just on describing the morphology of S. cameli and did not care about species differences [23].
The infection rate was higher in males (85.8%) than in females (81.0%), although this difference was not significant. This result corresponds with those reported by Woldemeskel and Gumi [26] and Shekarforoush et al. [30] who showed that the infection rate was independent of sex.
The inability to detect macroscopic cysts compared with the high prevalence of microscopic sarcocysts in the present study may be due to the fact that the dogs are the final host of Sarcocystis spp. in camels. Macroscopic cysts are of feline origin [37] and contact between camels and cats in this area is very rare as the nomadic owners never keep cats. Our findings confirmed the previous results reported by Hilali and Mohamed [2], Boid et al. [20], Dubey et al. [4], Fatani et al. [21], Latif et al. [25] in this respect who showed that the domestic dogs but not the cats act as the final host for the camel Sarcocystis.
In spite of the high incidence of camel Sarcocystis, little is known about its clinical and pathological effects. Except in severe infections, few symptoms were reported to be the outcome of this infection. However, the present observation indicates quite clearly that the Sarcocystis can account for a significant pathology. At first severe inflammatory, degenerative, and necrotic changes occur, leading to extensive damages of the musculoskeletal tissues. Later, the damaged parts undergo healing with fibrosis, scarring, and atrophy. These changes may occur concurrently in the same animal because of the prolonged irritation of the cysts.
Considering the facts that the infection can be massive, different organs may be involved and the cysts may remain in situ for a long time, it would not be too presumptuous to declare the condition as an important camel ailment warranting further detailed investigations. Because the impacts of camel Sarcocystis on the musculoskeletal function, feeding, health, and productivity of the animals, are not fully understood, it is necessary to study other aspects of this disease, especially its economical importance in the future.
ACKNOWLEDGEMENTS
The authors are grateful to Mr. Alireza Sadeghnia and Mr. Shahrokh Sarmadi from the Razi Vaccine and Serum Research Institute, and Mr. Homan Yazdanpour from the Pathobiology Department of The Veterinary School, Shiraz University for their technical assistance. They also thank the abattoir officials at Mashhad, Khorasan razavi Province, Iran for their helpful assistance.