Involvement of Src Family Tyrosine Kinase in Apoptosis of Human Neutrophils Induced by Protozoan Parasite Entamoeba histolytica
Article information
Abstract
Tyrosine kinases are one of the most important regulators for intracellular signal transduction related to inflammatory responses. However, there are no reports describing the effects of tyrosine kinases on neutrophil apoptosis induced by Entamoeba histolytica. In this study, isolated human neutrophils from peripheral blood were incubated with live trophozoites in the presence or absence of tyrosine kinase inhibitors. Entamoeba-induced receptor shedding of CD16 and PS externalization in neutrophils were inhibited by pre-incubation of neutrophils with the broad-spectrum tyrosine kinase inhibitor genistein or the Src family kinase inhibitor PP2. Entamoeba-induced ROS production was also inhibited by genistein or PP2. Moreover, genistein and PP2 blocked the phosphorylation of ERK and p38 MAPK in neutrophils induced by E. histolytica. These results suggest that Src tyrosine kinases may participate in the signaling event for ROS-dependent activation of MAPKs during neutrophil apoptosis induced by E. histolytica.
INTRODUCTION
Entamoeba histolytica, an enteric protozoan parasite, causes ulcerative lesions, dysentery and amebic liver abscess. E. histolytica is responsible for an estimated 50 million cases and 100,000 deaths worldwide each year [1]. E. histolytica trophozoites invade the intestinal epithelial cells and penetrate the underlying tissue, having close contact with inflammatory cells. E. histolytica trophozoites induce production of IL-8 by colonic epithelial cells, and C5a and C3a fragments produced by component on the parasite surface may also contribute to immune cell chemotaxis [1,2].
Neutrophils are the most abundant white blood cells and key components of the innate immune system. The main role of neutrophils during the acute inflammatory responses is to find and destroy invading microbial pathogens by phagocytosis, release of reactive oxygen species (ROS) and/or proteolytic enzymes [3]. Neutrophils are infiltrated in the inflammatory site at early stages of amebiasis [4]. Even though some studies in mice reported that neutrophils are not important to eliminate E. histolytica infection from the intestine [5], recent studies show that neutrophils are accumulated in colonic mucosa of patients with amebic colitis [6,7] and support that neutrophils play a vital role in resistance to amebic liver abscess and amebic colitis [4,8]. While host immune cells have various mechanisms for eliminating pathogens, E. histolytica has also developed defense strategies for its survival. E. histolytica trophozoites induce neutrophil apoptosis, which is associated with NADPH oxidase-generated ROS-mediated ERK1/2 activation [9,10]. Entamoeba-induced neutrophil apoptosis decreases tissue damage at the inflammatory lesions and is helpful for evading the host's immune responses. However, the signaling mechanism of neutrophil apoptosis induced by E. histolytica is not fully understood.
Tyrosine kinases are essential to regulate the inflammatory responses such as recruitment and activation of immune cells. Tyrosine kinases control many cellular processes, functioning to transduce signals within the neutrophil. The overall results of these signaling events in the neutrophil include F-actin polymerization, adhesion receptor priming, cell crawling, degranulation, and production of ROS [11-13]. In addition, tyrosine phosphorylation regulates the process of granulocyte apoptosis [14]. Although tyrosine kinases are important regulators of signal pathway, no previous investigators have reported on the role of tyrosine kinases in Entamoeba-induced neutrophil apoptosis. In this study, we investigated the role of Src family tyrosine kinase in neutrophil apoptosis induced by E. histolytica.
MATERIALS AND METHODS
Reagents and antibodies
The tyrosine kinase inhibitor genistein and PP2, and the NADPH oxidase inhibitor diphenyleneiodonium chloride (DPI) were purchased from MERCK (Darmstadt, Germany). PE-labeled mouse anti-human CD16 mAb and FITC-labeled annexin V were purchased from BD Pharmingen (Sandiego, California, USA). Fetal calf serum (FCS) and 2',7'-dichlorofluorescein-diacetate (DCF-DA) were purchased from Invitrogen (Carlsbad, California, USA). Rabbit polyclonal antibodies (Abs) against phospho-p38 MAPK, p38 MAPK, and phospho-ERK1/2 MAPK were obtained from Cell Signaling Technology (Danvers, Massachusetts, USA). Rabbit polyclonal antibody against ERK2 was obtained from Santa Cruz Biotechnology (Santa Cruz, California, USA). Unless stated otherwise, all other reagents were purchased from Sigma-Aldrich (St. Louis, Missouri, USA).
Cultivation of E. histolytica
E. histolytica strain HM1: IMSS was cultured axenically in glass screw-capped tubes using TYI-S-33 medium at 37℃ All amoebae used in this experiment were cultured for 48 hr and harvested by chilling on ice for 10 min followed by centrifugation at 200 g at 4℃ for 5 min. The resulting pellet was washed with RPMI 1640 medium containing 2 g/L NaHCO3, 50 mg/L gentamicin, 1 g/L human serum albumin, and 10% (v/v) heat-inactivated FCS, and was then resuspended in the culture medium. The amoebae were counted using a standard hemocytometer, and the viability, as judged by trypan blue exclusion test, was > 97%.
Neutrophil isolation and culture conditions for apoptosis
Human neutrophils were obtained from the heparinized venous blood of healthy volunteers. Briefly, 20 ml venous blood was diluted with an equal volume of PIPES buffer containing 25 mM PIPES, 50 mM NaCl, 5 mM KCl, 25 mM NaOH, and 5.4 mM glucose (pH 7.4). Mononuclear cells and platelets were removed by density gradient centrifugation over Histopaque with a density of 1.083 g/ml, and erythrocytes in the sediment were lysed by exposure to sterile distilled water on ice. The remaining granulocytes were washed with RPMI 1640 medium containing 1% FCS. The purified neutrophils of the bottom pellet were resuspended in the culture medium and kept on ice until use. The purity of neutrophils counted by May-Grünwald stain was > 94% on average. The viability of neutrophils using trypan blue exclusion test was > 99%. Isolated neutrophils were pre-incubated for 15 min at 37℃ with DMSO, 50 µM genistein or 30 µM PP2. After pre-incubation with inhibitors, neutrophils were washed once with culture medium. Neutrophils (4 × 105/200 µl) and E. histolytica trophozoites (4 × 104/200 µl) were combined into 48-well tissue culture plates to yield a final neutrophil: amoeba ratio of 10:1 and incubated at 37℃ for 30 min in a humidified CO2 incubator (5% CO2 and 95% air atmosphere). In all experiments, DMSO did not exceed 0.5% of culture medium, a concentration that did not affect ROS generation and neutrophil apoptosis induced by E. histolytica. Cytotoxicity was not observed by inhibitors at the tested concentrations.
Flow cytometric analysis for neutrophil apoptosis and intracellular ROS generation
Neutrophil apoptosis was quantified in 2 ways; the mean fluorescence intensity (MFI) of surface CD16 expression and the percentage of cells with annexin V bound on the cell surface, evaluated using PE-labeled anti-human CD16 antibody and FITC-labeled annexin V, respectively. Increase of the percentage of neutrophils with annexin V bound and the shedding of CD16 expression on the cell surface is known to be strongly correlated with apoptosis [10]. Intracellular ROS levels in neutrophils were measured using the fluorescence probe DCF-DA as described previously [15].
Western blotting
Neutrophils (1 × 106/group) were incubated for 5 min with or without E. histolytica trophozoites (1 × 105/group) in the presence or absence of tyrosine kinase inhibitors. After incubation, the reaction was stopped by brief centrifugation. The total protein was isolated from neutrophils using lysis (20 mM Tris-HCl, pH 7.5, 60 mM β-glycerophosphate, 10 mM EDTA, 10 mM MgCl2, 10 mM NaF, 2 mM DTT, 1 mM Na3VO4, 1 mM APMSF, 1% NP-40, and 5 µg/ml leupeptin) on ice for 30 min. After centrifugation at 12,000 g for 5 min, the supernatants were saved, diluted in SDS-PAGE loading buffer, and heated at 100℃ for 5 min. The samples were stored at -20℃ until use. Samples were subjected to 10% SDS polyacrylamide gels and were electrotransferred onto PVDF membranes. The membranes were blocked with 5% non-fat dry milk in 0.1% TBST at room temperature for 1 hr and then incubated with primary Abs against phosphorylated proteins of ERK1/2 or p38 MAPK at 4℃ overnight. After washing with TBST, the membranes were subsequently soaked with HRP-conjugated anti-rabbit IgG Abs at room temperature for 1 hr. Immunoreactivity was detected using LumiGLO (Cell Signaling Technology). Membranes were stripped by using stripping buffer (100 mM 2-ME, 2% SDS, 62.5 mM Tris-HCl, pH 6.7) at 56℃ for 30 min and probed for the corresponding Abs against non-phosphorylated MAPK proteins.
Statistics
The values in each graph represent means ± standard deviation (SD) of 4 independent experiments. Data were analyzed using student's t-test and values of P < 0.05 indicate statistical significance.
RESULTS
Pre-treatment with genistein or PP2 inhibits E. histolytica-induced apoptosis and ROS generation in human neutrophils
Incubation of human neutrophils with E. histolytica trophozoites induced receptor shedding of surface CD16 (2,288 ± 105 for medium vs. 855 ± 83 for Entamoeba) and increase of percentages of annexin V positive cells (21.1 ± 4.4 for medium vs. 42.1 ± 2.0 for Entamoeba). To check whether tyrosine kinases participate in Entamoeba-induced neutrophil apoptosis, we pre-incubated neutrophils with the broad tyrosine kinase inhibitor genistein or the Src family kinase inhibitor PP2 prior to addition of E. histolytica trophozoites. Genistein and PP2 attenuated Entamoeba-triggered reduction in the fluorescence intensity of surface CD16 expression (1,246 ± 95 for genistein; 1,258 ± 141 for PP2) and Entamoeba-triggered increase of percentages of annexin V positive cells (33.9 ± 1.6 for genistein; 36.3 ± 1.6 for PP2) (Fig. 1).
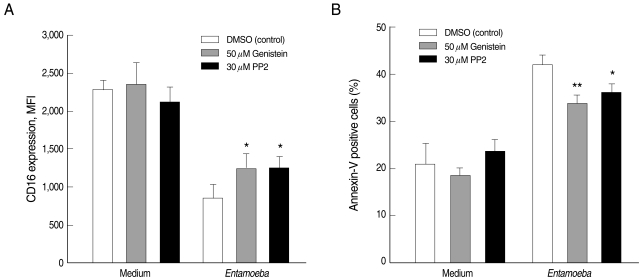
Effect of genistein and PP2 on the Entamoeba histolytica-induced neutrophil apoptosis. Neutrophils (4 × 105/well) were pre-incubated with 0.5% DMSO (control vehicle), 50 µM genistein, or 30 µM PP2 for 15 min and then incubated in the presence or absence of E. histolytica (4 × 104/well) for 30 min. After incubation, cells were stained with PE-labeled anti-human CD16 mAb (A) and FITC-labeled annexin V (B) for flow cytometric measurement of neutrophil apoptosis. The values in each graph represent the mean ± SD of 4 independent experiments. Significant differences from controls are as follows: *P < 0.05; **P < 0.01.
Previous reports have indicated that Entamoeba-induced neurtophil apoptosis is mediated by NADPH oxidase-derived ROS [10]. Neutrophils incubated with E. histolytica trophozoites showed a marked increase in DCF fluorescence (14.8 ± 6.3 for medium vs. 105.1 ± 25.0 for Entamoeba), which was suppressed by pre-incubation of neutrophils with genistein or PP2 (60.3 ± 26.3 for genistein; 49.5 ± 27.0 for PP2). As a positive control, the NADPH oxidase inhibitor DPI also effectively blocked Entamoeba-induced ROS production (Fig. 2).
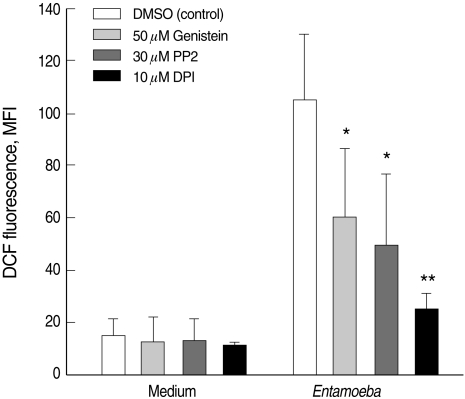
Effect of genistein and PP2 on the Entamoeba histolytica-induced ROS production in neurtophils. Neutrophils (4 × 105/well) were pre-incubated with 0.5% DMSO (control vehicle), 50 µM genistein, or 30 µM PP2 for 15 min and then incubated in the presence or absence of E. histolytica (4 × 104/well) for 10 min. After incubation, cells were stained with DCF-DA for flow cytometric measurement of ROS generation. The values in each graph represent the mean ± SD of 4 independent experiments. Significant differences from controls are as follows: *P < 0.05; **P < 0.01.
Pre-treatment with genistein or PP2 inhibits phosphorylation of ERK1/2 and p38 MAPK induced by E. histolytica
It has been reported that E. histolytica stimulates activation of ERK1/2 and p38 MAPK, which induces neutrophil apoptosis [10]. We examined the role of tyrosine kinases in Entamoeba-induced ERK1/2 and p38 MAPK phosphorylation in neutrophils. In accordance with previous results, E. histolytica induced phosphorylaiton of ERK1/2 and p38 MAPK in neutrophils. The ERK1/2 and the p38 MAPK activation induced by E. histolytica were also inhibited by pre-incubation of neutrophils with 50 µM genistein and 30 µM PP2, respectively (Fig. 3).
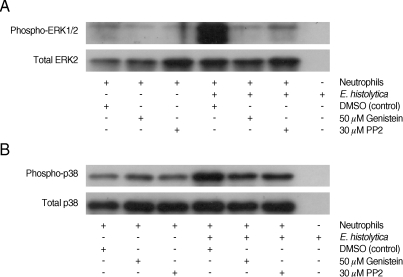
Effect of genistein and PP2 on the Entamoeba histolytica-induced phosphorylation of ERK-1/2 and p38 MAPK in neutrophils. Neutrophils (1 × 106/well) were pre-incubated with 0.5% DMSO (control vehicle), 50 µM genistein, or 30 µM PP2 for 15 min and then incubated in the presence or absence of E. histolytica (1 × 105/well) for 5 min. Cell lysates were separated by SDS-PAGE and blotted with anti-phospho-ERK1/2 (A) and anti-phospho-p38 (B).
DISCUSSION
Protein tyrosine kinases consist of 2 groups; intracellular domain of receptors (receptor tyrosine kinases) and cytosolic enzymes (non-receptor tyrosine kinases) [16]. Src kinase family is categorized into non-receptor tyrosine kinases that have broad functions and interact with other signaling mechanisms intracellularly [11]. Src tyrosine kinases are one of the most critical families for intracellular signal transduction related to acute inflammatory responses. It is essential for the recruitment and activation of various immune cells [17]. The function of tyrosine kinases in signal transduction of Entamoeba-induced neutrophil apoptosis has not been analyzed. Therefore, broad tyrosine kinase inhibitor genistein and Src family kinase inhibitor PP2 were used to define the role of specific kinases in apoptotic signaling of neutrophils by E. histolytica.
In the present study, we have demonstrated that activation of Src family kinase play an important role in the process of neutrophil apoptosis induced by E. histolyitca. For example, pro-apoptotic effect of E. histolytica on neutrophils was partially, but significantly, suppressed by the pre-incubation of neutrophils with Src family kinase inhibitors such as genistein and PP2. In addition, genistein and PP2 inhibited Entamoeba-triggered ROS production in neurtophils (42.6% inhibition for genistein and 52.9% inhibition for PP2). One of the characteristic features in neutrophil apoptosis induced by E. histolytica has been reported to be activation of MAPK family, including ERK1/2 and p38 MAPK [10]. A previous work has also shown that Src family kinase is associated with p38 MAPK activation. For example, fMLP-induced neutrophil degranulation via p38 MAPK activation was blocked by a Src family kinase inhibitor [18]. In the present study, Entamoeba-induced phosphorylation of ERK1/2 as well as p38 MAPK were nicely blocked by pretreatment of neutrophils with genistein or PP2. Taken together, our results suggest that ROS-mediated activation of ERK1/2 as well as p38 MAPK during neutrophil apoptosis induced by E. histolytica might be dependent upon activation of the Src family kinase.
β2-integrin, the most abundant integrin on neutrophils, transduces signals into the cell and contributes to apoptosis in inflammation [19]. Our previous studies showed that neurophil apoptosis by E. histolytica is contact-dependent and is mediated by interaction through β2-integrin receptor [15]. Src family kinase is activated during binding of ligand to β2-integrin on neutrophil [20]. Lowell et al. showed that neutrophils from Hck-/-, Fgr-/-, the main Src family members, double mutant mice failed to integrin-dependent respiratory burst [21]. Neurtophils deficient of Src family kinases also showed impaired activation of spleen tyrosine kinase Syk [22], an important downstream signaling component of β2-integrins, which mediates cellular functions such as redistribution of the actin cytoskeleton, ROS production, and degranulation [23,24]. β2-integrin-mediated ROS generation and neutrophil apoptosis by E. histolytica was reduced by pre-treatment with PI-3-kinase inhibitor wortmannin [15]. Previous studies demonstrate that the p85 regulatory subunit of PI-3-kinase interacts with Src family kinase or Syk [25,26]. The signaling through leukocyte integrins regulates tyrosine kinase activities, which lead to assembly of NADPH oxidase components in an active form [27]. Therefore, our results showing that genistein and PP2 inhibit Entamoeba-induced ROS production suggest that β2-integrin-triggered respiratory burst in neutrophils by E. histolytica might proceed through PI-3-kinase activated by tyrosine kinases. The possible involvement of Syk in the above processes requires further investigation.
In conclusion, this report shows for the first time that Src family kinase is a key mediator of ROS-mediated neutrophil apoptosis by E. histolytica. Recently, many tyrosine kinase inhibitors have been developed and used as therapeutic target for treatment of many inflammatory diseases and malignancies [28,29]. Elucidating the details of this tyrosine kinase-dependent signaling mechanism in Entamoeba-induced neutrophil apoptosis will provide the groundwork to better understand pathogenesis of invasive amebiasis in humans.
ACKNOWLEDGEMENTS
This work was supported by the faculty research fund of Konkuk University in 2007.