Calpains are Involved in Entamoeba histolytica-Induced Death of HT-29 Colonic Epithelial Cells
Article information
Abstract
Entamoeba histolytica is an enteric tissue-invading protozoan parasite that can cause amebic colitis and liver abscess in humans. E. histolytica has the capability to kill colon epithelial cells in vitro; however, information regarding the role of calpain in colon cell death induced by ameba is limited. In this study, we investigated whether calpains are involved in the E. histolytica-induced cell death of HT-29 colonic epithelial cells. When HT-29 cells were co-incubated with E. histolytica, the propidium iodide stained dead cells markedly increased compared to that in HT-29 cells incubated with medium alone. This pro-death effect induced by ameba was effectively blocked by pretreatment of HT-29 cells with the calpain inhibitor, calpeptin. Moreover, knockdown of m- and µ-calpain by siRNA significantly reduced E. histolytica-induced HT-29 cell death. These results suggest that m- and µ-calpain may be involved in colon epithelial cell death induced by E. histolytica.
Entamoeba histolytica is an enteric protozoan parasite that can cause amebic dysentery and occasional liver abscess in humans. During infection, amebic trophozoites released from cysts bind colonic mucins, causing subsequent mucin degradation and cell death of colonic epithelial cells in a contact-dependent manner [1,2]. Recent reports have indicated that E. histolytica trophozoites can kill various kinds of host cells via apoptosis, including neutrophils [3], T cells [4], and macrophages [5]. In addition, several lines of evidence now show that unique signaling mechanisms are involved in the process of host cell apoptosis induced by E. histolytica. In particular, calpain, a calcium-dependent cysteine protease, has been reported to be responsible for activation of caspase-3 and protein tyrosine phosphatase in E. histolytica-induced DNA fragmentation and dephosphorylation in Jurkat T cells [6,7]. Moreover, treatment of cells with a calcium channel blocker (verapamil) and chelators, ethylenediaminetetraacetic acid (EDTA) and ethylene glycol-bis (2-aminoethylether)- N,N,N',N'-tetraacetic acid (EGTA), results in a marked reduction of E. histolytica-induced cell death [8]. These results suggest that calpain may play a crucial role in pro-apoptotic signaling in host cells following exposure to E. histolytica. However, it is unknown whether calpains are involved in cell death of colon epithelial cells induced by E. histolytica.
Calpains are cytosolic proteases of which numerous isoforms have been identified in organisms ranging from mammals to invertebrates. Among the numerous calpain isoforms, m- and µ-calpain (conventional calpains) are ubiquitously expressed and are regulated by micromolar and millimolar increases in intracellular Ca2+ levels, respectively [9]. They are also the most extensively analyzed calpain isoforms and have been shown to play roles in various physiological functions, including cell motility [10], migration [11] apoptosis [12,13], cell growth [14], and cell cycle progression [15]. In this study, using inhibitor and small interference RNA (siRNAi), we investigated the roles of m- and µ-calpain in the E. histolytica-induced cell death of HT-29 colon epithelial cells.
Trophozoites of E. histolytica (HM1: IMSS strain) were grown axenically in a glass screw-capped tube containing TYI-S-33 medium at 37℃. After cultivation for 48-72 hr, the trophozoites in logarithmic phase growth were harvested via incubation on ice for 10 min followed by centrifugation at 200 g at 4℃ for 5 min. For co-incubation with HT-29 cells, the trophozoites were washed with RPMI 1640 medium supplemented with 2 g/L NaHCO3, 50 mg/L gentamicin, 1 g/L human serum albumin, and 10% (v/v) heat-inactivated FBS and were suspended in culture media. The viability of the amoebae, as judged by trypan blue dye exclusion tests, was always 100%. HT-29 colon epithelial cells (American Type Culture Collection, Manassas, Virginia, USA) were maintained in RPMI 1640 medium containing 10% FBS, penicillin (100 U/ml), and streptomycin (100 µg/ml) and were incubated at 37℃ for 30 or 60 min in a humidified CO2 incubator (5% CO2 and 95% air atmosphere). HT-29 cells were pretreated with or without a calpain inhibitor calpeptin (1 mM) (Calbiochem, La Jolla, California, USA) for 30 min at 37℃. After preincubation, HT-29 cells were washed once with culture medium before being suspended with E. histolytica. The designed sequences of the small interfering RNAs (siRNAs) specific for human large subunits of m-calpain and µ-calpain were 5'-CCAGGACUACGAGGCGCUGdTdT-3' and 5'-GCUAGUGUUCGUGCACUCUdTdT-3', respectively. The nucleotide RNA-DNA chimeric duplexes and control siRNA (Dharmacon Cat# D-001210-02-20) were obtained from Dharmacon (Seoul, Korea).
Cells were transfected with various concentrations of siRNA and Lipofectamine™ reagent (Invitrogen, Cergy Pontoise, France) for 72 hr before E. histolytica treatment according to the manufacturer's instructions. Depletions of endogenous m-calpain and µ-calpain by siRNA treatment were confirmed using western blotting. In mock transfections, all reagents were used except for siRNA. HT-29 cells throughout the experiments were viable as determined by the trypan blue exclusion assays (data not shown). At 72 hr post-transfection, the efficiency of siRNA (80 nM) knockdown of calpain was confirmed via western blotting using µ-calpain- and m-calpain-specific antibodies (Cell Signaling Technology, Beverly, Massachusetts, USA), with a β-actin specific antibody (Calbiochem) serving as an equal loading control. At 72 hr post-transfection, samples containing 30 µg of total protein were resolved using 12% SDS-PAGE, transferred onto nitrocellulose membranes (Bio-Rad, Hercules, California, USA) via electroblotting, and probed with specific antibodies. Blots were developed using an ECL kit (Intron, Seoul, Korea) and detected using chemiluminescence (LAS1000). HT-29 cell death was determined after cell staining with propidium iodide (PI) (BD Pharmingen, San Diego, California, USA) using flow cytometry (Becton Dickinson, California, USA). Flow cytometric analysis for PI-stained cells was performed on at least 10,000 cells, and data analyses were performed with Cell Quest software. HT-29 cells and E. histolytica were distinguished on the basis of differences in forward and side scatter characteristics. Results are expressed as the mean±SEM from three independent experiments. Statistical analysis was performed using paired Student's t-test. The value of P<0.05 was considered statistically significant.
We investigated the role of calpain in HT29 cell death induced by E. histolytica. As shown in Fig. 1, E. histolytica-induced HT29 cell death was effectively blocked by pretreatment of cells with the calpain inhibitor, calpeptin, suggesting that activation of calpain in host cells contributes to HT29 cell death induced by E. histolytica. To address the role of calpain activity in E. histolytica-induced HT-29 cell death, we designed siRNA to selectively knockdown the expressions of calpains. Because the 2 ubiquitous calpain isoforms, m- and µ-calpain, are functional only when associated with a small common regulatory subunit [9], we transfected host cells with specific siRNA to specifically knockdown either m- or µ-calpain, knockdown respectively. Preliminary experiments with siRNA were performed to determine optimum dose and incubation time by examining siRNA concentrations of 20, 40, 80, or 160 nM for 24, 48, or 72 hr. A siRNA concentration of 80 nM for 72 hr resulted in maximum inhibition of protein expression without affecting HT-29 cell viability (data not shown). Therefore, HT-29 cells were transfected for 72 hr with 80 nM siRNA specific for m-calpain or µ-calpain.
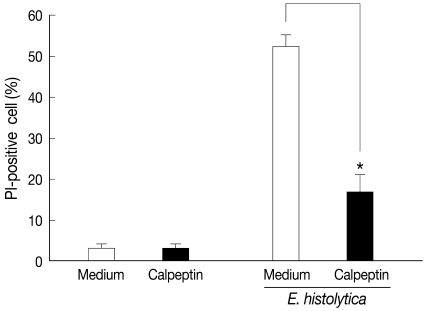
Pretreatment with calpeptin inhibits HT-29 colon cell death induced by E. histolytica. HT-29 cells (4×106/sample) were pretreated with calpeptin (1 mM) for 30 min at 37℃. After pre-incubation, HT-29 cells were washed once with culture medium before incubation with E. histolytica for 60 min at a ratio of 10:1. Cell death was determined through cell staining with propidium iodide (PI). Data are expressed as the mean±SEM from 4 independent experiments.
After 72 hr post-transfection, the cells were co-incubated for 60 min with E. histolytica. As shown in Fig. 2A, transfection with m- or µ-calpain siRNA reduced protein levels. Mock transfection and transfection of 80 nM control (non-targeting) siRNA in HT-29 cells did not affect protein levels of m- or µ-calpain (Fig. 2A). Notably, neither m-calpain siRNA or µ-calpain siRNA affected the protein level of the calpain isoform (Fig. 2A). Down regulations of m- and µ-calpain by siRNA significantly prevented E. histolytica-induced HT-29 cell death (65% inhibition). In contrast, mock transfection and transfection with control non-targeting siRNA in HT-29 cells did not prevent cell death (Fig. 2B).
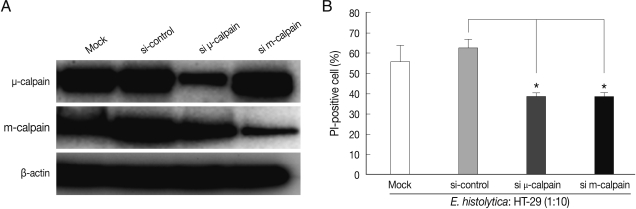
siRNA-mediated knockdown of m-calpain or µ-calpain inhibits E. histolytica-induced cell death in HT-29 colon epithelial cells. (A) Analysis of m- or µ-calpain protein levels using immunoblotting following knockdown of calpain by siRNA in HT-29 cells. At 72 hr post-transfection, whole-cell lysates from HT-29 cells transfected with vehicle alone (Mock), control scrambled siRNA (80 nM), or m or µ-calpain siRNA (80 nM) were subjected to immunoblotting with Ab to m- or µ-calpain. β-actin was used as an equal loading control. (B) Effects of m- or µ-calpain siRNA on E. histolytica-induced cell death in HT-29 cells. At 72 hr post-transfection, HT-29 cells (4 × 106) transfected with vehicle alone (Mock), control scrambled siRNA or m- or µ-calpain siRNA were incubated with E. histolytica (4 × 105) for 1 hr. Host cell death was measured using PI staining for flow cytometry analysis. Data are expressed as the mean±SEM of 3 experiments.
It has been reported that E. histolytica triggers activation of host calpain in Jurkat T cells, which is known to be closely involved in the cleavage of various host proteins associated with cell signaling and regulation, including tyrosine phosphatases (PTPs), such as PTP1B [6], SHP-1 and SHP-2 [16], and caspase-3 and calpastatin [17]. Thus, activation of host calpain might lead to accelerate host cell death. In our previous study, pretreatment of Jurkat cells with the calpain inhibitor calpeptin effectively blocked E. histolytica-triggered cleavage of caspases-3, -6, and -7 during cell death [7], although inhibition of calpain activity in Jurkat T cells with calpeptin did not effectively inhibit host cell death induced by E. histolytica. In the present study, however, in the context of HT-29 colon cell death induced by E. histolytica, pretreatment with calpeptin or knockdown of m- or µ-calpain protein expression in HT-29 cells by specific siRNA efficiently prevented E. histolytica-induced cell death. Taken together, our results strongly suggest that host m- and µ-calpain may play crucial roles in E. histolytica-induced colon epithelial cell death during the early phase of intestinal amebiasis. E. histolytica-induced apoptotic cell death in neutrophils and T cells through caspase-3 activation might lead to chronic infection. Like macrophages, it is interesting to note that amoebae themselves can silently scavenge apoptotic Jurkat T cells via the phosphatidyl serine (PS) receptor on the amebic surface [17] to prevent secondary necrosis of host cells. These results suggest that both the host and amoebae may attempt to exist together with solicitude through a delicate cross talk between immune cells and the parasite when amoebae invade the tissues by breaking intestinal epithelial layers.
In conclusion, we have demonstrated that m-and µ-calpain are involved in the context of cell death in HT-29 colon epithelial cells induced by E. histolytica. These results will help to improve our understanding of ameba-induced tissue inflammation during human amoebiasis [18].
ACKNOWLEDGEMENTS
This study was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2006-311-E00256).