Changing Patterns of Acute Phase Proteins and Inflammatory Mediators in Experimental Caprine Coccidiosis
Article information
Abstract
This experiment was conducted to assess the changing patterns and relative values of acute phase proteins and inflammatory cytokines in experimental caprine coccidiosis. Eighteen newborn kids were allocated to 3 equal groups. Two groups, A and B, were inoculated with a single dose of 1×103 and1×105 sporulated oocysts of Eimeria arloingi, respectively. The third group, C, received distilled water as the control. Blood samples were collected from the jugular vein of each kid in both groups before inoculation and at days 7, 14, 21, 28, 35, and 42 post-inoculation (PI), and the levels of haptoglobin (Hp), serum amyloid A (SAA), TNF-α, and IFN-γ were measured. For histopathological examinations, 2 kids were selected from each group, euthanized, and necropsied on day 42 PI. Mean Hp concentrations in groups A and B (0.34 and 0.68 g/L) at day 7 PI were 3.2 and 6.3 times higher than the levels before inoculation. The mean SAA concentrations in groups A and B (25.6 and 83.5 µg/ml) at day 7 PI were 4.2 and 13.7 times higher than the levels before inoculation. The magnitude and duration of the Hp and SAA responses correlated well with the inoculation doses and the severity of the clinical signs and diarrhea in kids. These results were consistent with the histopathological features, which showed advanced widespread lesions in group B. In both groups, significant correlations were observed for TNF-α and IFN-γ with SAA and Hp, respectively. In conclusion, Hp and SAA can be useful non-specific diagnostic indicators in caprine coccidiosis.
INTRODUCTION
Acute phase proteins (APPs) are a group of blood proteins that show concentration increases (positive APP) or decreases (negative APP) after inflammation, infection, trauma, some neoplastic growth, or immunological disorders. Early reaction of the host to infection or tissue damage leads to a release of pro-inflammatory cytokines [1]. The main cytokines released by infected cells are primarily inteferons, especially IFN-γ, from mononuclear inflammatory cells, although TNF-α and IL-1β may also be involved. The positive APPs are mainly C-reactive protein (CRP), Hp, and SAA which are released by the hepatocytes after cytokine stimulation [2]. When severe cellular destruction is present, a full acute phase response (APR) can be observed [2,3]. The APR is considered to be a non-specific immune mechanism involving systemic and metabolic changes against insult before specific immunity is achieved [4, 5]. During the acute phase response, the serum concentration of the APPs changes dramatically. The circulating concentrations of the APPs are related to the severity of the disorder and the extent of tissue damage in the affected animals. APP levels are not suitable for establishing a specific diagnosis, but quantification of their concentration can provide objective information about the extent of ongoing lesions in individual animals [6, 7]. Ruminant coccidiosis is a contagious protozoal disease that occurs especially in lambs and kids, with a worldwide distribution [8].
Clinical coccidiosis occurs mainly in 4-6-month old kids and lambs and stress factors, such as inclement weather, weaning, dietary changes, traveling, and regrouping have important roles in caprine coccidiosis [9]. In goats, 16 species have been described, of which Eimeria christenseni, Eimeria arloingi, Eimeria caprina, and Eimeria ninakohlyakimovae are of major concern [10-12]. E. arloingi, a common coccidian species of goats worldwide, causes high mortality in kids and leads to economic loss from both subclinical and clinical infections, including low growth performance, decrease in productivity, and treatment cost [13, 14].
The diagnosis of coccidiosis depends on the clinical findings (diarrhea, dehydration, and progressive emaciation), the presence of large numbers of oocysts in the feces, and appropriate signalment and intestinal lesions at necropsy [8]. Acute phase proteins may provide alternative means of monitoring animal health. An increased focus on the application of APPs for this purpose has been developed [15]. APPs may be useful for providing information about the stage of clinical and subclinical infections and also for prognosis of their severity.
To our knowledge, there is no document describing the changes in serum concentrations of APPs and inflammatory mediators in caprine coccidiosis. The purpose of this study was to evaluate the dependent changes in the serum concentrations of Hp, SAA, TNF-α, and IFN-γ in kids experimentally infected by E. arloingi.
MATERIALS AND METHODS
Experiment, animals, and sampling
A pure strain of E. arloingi was obtained from the Parasitology Department of Shiraz University, Shiraz, Iran. The oocysts were stored at 4℃ in 2.5% potassium dichromate (K2Cr2O7) solution for about 2 weeks before their use in the experiment. Before inoculation, the suspension containing oocysts was freed of the potassium dichromate by series washing and centrifugation at 750 g for 5 min. The sediment was mixed with 100 ml distilled water and the number of oocysts was calculated for each ml of suspension. Eighteen Iranian crossbred kids were separated from their dams immediately after birth, fed with a milk replacer and reared under coccidia-free conditions in closed rooms with restricted access. At 15 days of age, the kids were divided into 2 groups. The kids of groups A (n=6) and B (n=6) were infected orally with 1×103 (low dose) and 1×105 (high dose) sporulated oocysts of E. arloingi per animal, respectively. The third group, C, received distilled water as the control.
Fresh fecal samples, approximately 3-5 g, were obtained directly from the rectum by stimulation of the anus and were examined for the presence of oocysts every day. Fecal consistency was assessed on the basis of a scoring system (1, normal; 2, semi liquid to liquid; 3, watery; 4, hemorrhagic and/or with tissue). Blood samples for determination of serum Hp, SAA, IFN-γ, and TNF-α were collected from the jugular vein of each kid in both groups before inoculation and at days 7, 14, 21, 28, 35, and 42 PI. Simultaneously, blood samples were collected from 6 non-infected kids as controls. The sera were separated following centrifugation for 10 min at 750 g and were stored at -20℃ until analyzed.
Measurements
Haptoglobin (Hp) was measured based on prevention of the peroxidase activity of hemoglobin, which is directly proportional to the amount of Hp. The analytical sensitivity of this test in serum has been determined as 0.0156 mg/ml for Hp by the manufacturer (Tridelta Development Plc, Wicklow, Ireland).
Serum amyloid A (SAA) was measured by a solid phase sandwich-ELISA. The analytical sensitivity of this test in serum has been determined as 0.3 µg/ml for SAA by the manufacturer (Tridelta Development Plc). IFN-γ and TNF-α were measured by a solid phase sandwich-ELISA (AbC 606 and AbC 607, respectively; Votre Fournisseur AbCys S.A., Paris, France).
Histopathological examinations
For histopathological examinations, 2 kids were selected from each group, euthanized, and necropsied at day 42 PI. After systematic postmortem examination, the small and large intestines were opened and inspected carefully. Appropriate samples of the visceral organs including small and large intestines, liver, spleen, pancreas, and mesenteric lymph nodes from 4 affected kids were fixed in 10% neutral buffered formalin. Paraffin-embedded tissues were sectioned at 5 µm thickness, stained with hematoxylin-eosin, and examined with a light microscope.
Statistical analysis
Descriptive statistics, including the mean, standard deviation, median, minimum, and maximum were calculated for all variables. The ANOVA and Tukey tests were used for comparison of different parameters. Data were analyzed by SPSS software, version 16, and P<0.05 was accepted as a statistically significant difference.
RESULTS
All animals in both groups showed the symptoms of clinical coccidiosis. The oocyst shedding was observed after the prepatent period, which varied between 16 to 18 days in groups A and B. All inoculated kids produced comparable numbers of oocysts (Fig. 1). The clinical signs of the infected kids varied with the size of the inoculation dose. The signs in group B were more severe. In group A, diarrhea started from day 20 PI, a kid with semiliquid and the others with liquid diarrhea (score 2). Severe diarrhea with variable amounts of mucus (score 3) occurred in group B from day 18 PI. Other signs included dehydration, anorexia, depression, paleness of eye mucosa, and listlessness.
The median pre-inoculation concentration of Hp was 0.1 g/L, and a significant increase was detected at day 7 PI (Fig. 2A). The highest median concentration (0.47 and 0.7 g/L) was detected at days 14 and 21 PI in groups A and B, respectively. The levels of this protein remained increased until the end of the experiment.
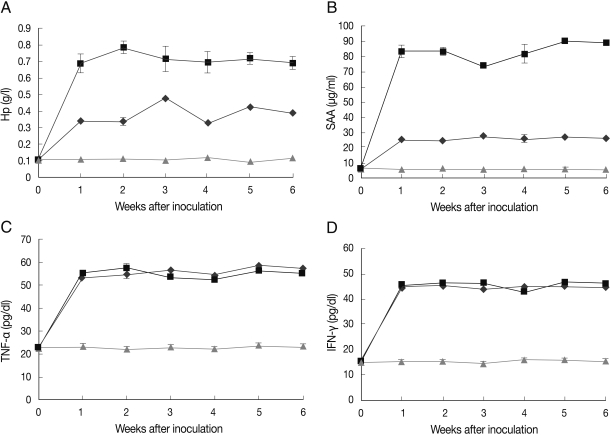
The kinetics of acute phase proteins and inflammatory mediators following experimental infection of goats (kids) with E. arloingi. (A) Haptoglobin (Hp), (B) Serum amyloid A (SAA), (C) TNF-α, and (D) IFN-γ. Results are shown as mean±SEM. 0=before inoculation (♦, Group A; ▪, Group B; ▴, Group C).
The median SAA concentration prior to inoculation was 6.1 µg/ml, and concentrations of this protein were significantly increased at day 7 PI (Fig. 2B). The maximum median SAA concentration (27.8 and 90.4 µg/ml) was reached at days 35 and 21 after inoculation in groups A and B, respectively. The levels of this protein remained increased until the end of the experiment.
The mean TNF-α concentration before inoculation was 22.7 pg/dl, and a significant increase (about 2.4 times) was observed at day 7 PI (Fig. 2C). The highest median concentrations were 57.6 pg/dl in group A and 58.5 pg/dl in group B. The mean IFN-γ concentration before inoculation was 15.3 pg/dl, and a significant increase (about 3 times) was observed at day 7 PI (Fig. 2D). The highest median concentrations were 46.7 pg/dl and 45.8 pg/dl in groups A and B, respectively.
There were significant correlations between Hp and SAA (r=0.949, P<0. 01), Hp with TNF-α(r=0.742, P<0. 01), Hp with IFN-γ(r=0. 728, P<0. 01), SAA with TNF-α(r=0.635, P<0.01) and SAA with IFN-γ(r=0.627, P<0.01) in both groups. Significant correlation was observed for TNF-α and IFN-γ(r=0.961, P<0.01). As shown in Fig. 2A and 2B, Hp and SAA concentrations were significantly higher in group B compared with group A (P<0.05) in all weeks. However, there were no significant changes in concentrations of TNF-α and IFN-γ between group A and group B. During the experiment, in group C, the concentrations of Hp, SAA, TNF-α, and IFN-γ were within the reference interval.
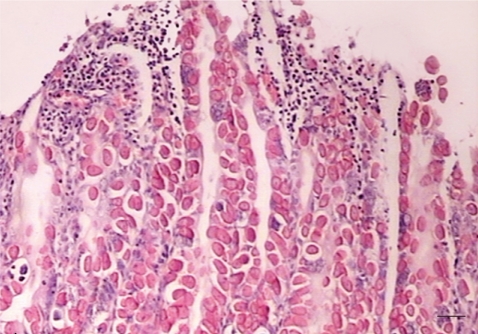
There were differences between groups A and B with regards to gross and histopathological lesions. The most remarkable macroscopic lesion was white to grey, slightly to well-raised pin-point foci to relatively large nodules mostly on affected mucosa in the middle jejunum to the ileum (Fig. 3). The kids in group A had milder lesions, including a few scattered whitish non-pedunculated nodules in the mucosa of the jejunum and ileum. The kids in group B showed advanced widespread lesions characterized by thickened mucosa due to mucosal hyperplasia and adenomatous-like changes in the mucosa and a cerebriform or gyrate pattern of projections visible from the serosa.

Jejunum of an infected kid (formalin-fixed). Thickening of the jejunal mucosa and the presence of scattered whitish pinhead size (1-2 mm in diameter) nodules (arrows) are seen.
The main histopathological lesion in both groups was proliferative enteritis associated with the presence of the developmental stages of parasite including mature schizonts, microgametocytes, macrogametocytes, and immature oocysts in the epithelial cells of villi and crypts of the jejunum, ileum, cecum, and proximal colon (Fig. 4). Also, mild lymphoid hyperplasia of the Peyer's patches, remarkable congestion, and edema were observed in some cases. Generally, the kids in group B showed more prominent microscopic lesions than those in group A.
DISCUSSION
Caprine coccidiosis is an important disease resulting from complex interactions between parasites and host, with many factors influencing the severity of the disease. Age, stress, genetic susceptibility, physical condition, and the degree of immunity are important in pathogenesis [10, 16, 17]. The development of clinical coccidiosis in goats is considerably dependent on the species involved [18]. Coccidiosis causes high morbidity and mortality in livestock, and diagnosis is limited to clinical findings, the presence of large numbers of oocysts in the feces, and histopathological findings [8].
There are relatively few reports of APP induction by parasite infections, although infections with Plasmodium falciparum, Babesia canis, Leishmania infantum, and Trypanosoma brucei might be accompanied by increased levels of APPs [19-23]. Haptoglobin and SAA are major acute phase proteins in most species studied [24]. In our experiment, we have shown that the APP response is induced in kids following infection with E. arloingi. The exact mechanism whereby E. arloingi causes APR in kids remains unclear. However, the induction of APPs supports the hypothesis that pro-inflammatory cytokines play an important role [25].
The acute phase response is regulated by a complex network of interactions [5,25]. The main pathway leading to APP production involves the initial release of pro-inflammatory cytokines by macrophages and other inflammatory cells at the site of inflammation or infection. The most important inducers of APPs are cytokines of the IL-1, TNF, and IL-6 families. TNF-α, IL-1β, and IFN-γ are essential for induction of other cytokines (IL-6 and IL-8) and agents, such as platelet activating factor, prostaglandins, leukotrienes, and nitric oxide. When these pro-inflammatory cytokines spill into the circulation, they induce the release of APPs by the liver into the circulation. IL-6 is the principal regulator of most APPs and induces the production of Hp, whereas IL-1 and TNF-α induce expression of SAA and α1-acid glycoprotein and Hp, depending on the mammalian species [1,25].
As was demonstrated in this study, serum Hp, SAA, TNF-α, and IFN-γ concentrations were significantly higher on day 7 after inoculation compared with their concentrations before inoculation, and the levels of these proteins remained increased until the end of the experiment. Hp is a major APP in ruminants, in which species it has a slight circulating level in normal animals, but increases over 100 folds on stimulation [26]. The mean Hp concentrations in groups A and B at day 7 PI were 3.2 and 6.3 times higher than the levels before inoculation. In both groups, significant correlations were observed for Hp with SAA, TNF-α, and IFN-γ. Petersen et al. [27] reported that clinical signs of lameness, diarrhea, respiratory disease, and ear necrosis were reflected in a high serum Hp concentration. Many studies have reported the serum Hp as a clinically useful parameter for measuring the occurrence and severity of inflammatory responses in cattle with enteritis, peritonitis, endometritis, mastitis, pneumonia, endocarditis, abscesses, and other natural or experimental infectious conditions [15,28-37].
The mean SAA concentrations in groups A and B at day 7 PI were 4.2 and 13.7 times higher than the levels before inoculation. In both groups, significant correlations were observed for SAA with Hp, TNF-α, and IFN-γ. SAA is a remarkably moderate acute phase protein in cattle increasing around 2-5 times during an acute phase response [38-40]. Because of the difficulty of measuring serum SAA levels, the application of SAA assays to veterinary diagnosis has not been as widespread as that of Hp assays. SAA has been suggested to be more useful in distinguishing acute and chronic inflammations than neutrophil counts and white blood cell counts [41]
In our study, the magnitude and the duration of the Hp and SAA responses correlated well with the inoculation doses and severity of the clinical signs and diarrhea in individual animals. Hp and SAA concentrations were significantly higher in group B compared with group A (P<0.05). These results were consistent with histopathological investigations, which showed advanced widespread lesions in group B. In some papers, no correlation between Hp and disease severity was observed [34,36].
Sickness behavior with decreased appetite or anorexia is mediated by the pro-inflammatory cytokines through induction of prostaglandins [1,42,43]. The elevation in APPs following E. arloingi infection very strongly indicated that pro-inflammatory cytokines were released into the peripheral circulation. Significant increases in TNF-α and IFN-γ concentrations (about 2.4 and 3.0 times, respectively) were observed at day 7 PI in both groups. Significant correlations were observed for TNF-α and IFN-γ with Hp and SAA in both groups. Although serum concentrations of TNF-α and IFN-γ increased after inoculation in both groups, significant correlations were not observed with the inoculation doses and severity of the clinical signs and diarrhea in individual animals. It is important to note that the APP-response-inducing cytokines are small molecules with a very short half-life. Therefore, cytokines are not very useful for most general diagnostic purposes, in contrast to the APPs, which are stable proteins and have relatively long half-lives [38,44]. In addition, assays for the individual pro-inflammatory cytokines, TNF-α, IL-1, and IL-6 are not commercially available for animals.
According to our study, it seems that SAA and Hp are 2 major APPs that significantly increase in caprine coccidiosis. Considering the timing of the response and the correlation between the magnitude of the response and the severity of the infection, it can be established that parasites causing focal lesions can induce acute phase responses, most probably indirectly via parasite-mediated tissue perturbations.
In conclusion, measuring Hp and SAA can be a suitable indicator of inflammatory reactions in caprine coccidiosis. In our experiment, increased concentrations of APPs were observed before the appearance of clinical signs. Therefore, APPs could be used as a non-specific marker for disease as well as for monitoring the response to treatment in coccidiosis. Measuring APPs along with clinical signs and oocysts per gram (OPG) can be useful for providing information about the stages of clinical and subclinical coccidiosis.