Naegleria fowleri Lysate Induces Strong Cytopathic Effects and Pro-inflammatory Cytokine Release in Rat Microglial Cells
Article information
Abstract
Naegleria fowleri, a ubiquitous free-living ameba, causes fatal primary amebic meningoencephalitis in humans. N. fowleri trophozoites are known to induce cytopathic changes upon contact with microglial cells, including necrotic and apoptotic cell death and pro-inflammatory cytokine release. In this study, we treated rat microglial cells with amebic lysate to probe contact-independent mechanisms for cytotoxicity, determining through a combination of light microscopy and scanning and transmission electron microscopy whether N. fowleri lysate could effect on both necrosis and apoptosis on microglia in a time- as well as dose-dependent fashion. A 51Cr release assay demonstrated pronounced lysate induction of cytotoxicity (71.5%) toward microglial cells by 24 hr after its addition to cultures. In an assay of pro-inflammatory cytokine release, microglial cells treated with N. fowleri lysate produced TNF-α, IL-6, and IL-1β, though generation of the former 2 cytokines was reduced with time, and that of the last increased throughout the experimental period. In summary, N. fowleri lysate exerted strong cytopathic effects on microglial cells, and elicited pro-inflammatory cytokine release as a primary immune response.
Free-living N. fowleri is a ubiquitous, soil- and water-borne ameba that can cause fatal primary amebic meningoencephalitis (PAM) in both humans and animal models. PAM occurs predominantly in healthy young adults and adolescents and in non-immunocompromised children [1]. PAM patients generally have a history of swimming or recreation in ponds or hot-springs, or on river banks [2-4]. The development of PAM results from the invasion of trophozoites from ameba-contaminated water into the nasal mucosa and their passage via the olfactory nerves to the forebrain, followed by multiplication in the tissues of the central nervous system (CNS) [5,6]. However, the precise mechanisms by which they exert their pathogenicity are unknown. Previous researches have suggested that infection may evoke both contact-dependent and contact-independent CNS pathology [7-11].
The mechanics of contact-dependent N. fowleri pathogenicity were previously observed to proceed via formation of vigorous pseudopodia and food-cup structures by trophozoites incubated with varying target cell-types [7,8]. Murine microglial cells experienced both necrotic and apoptotic cell death when exposed to N. fowleri trophozoites, with amebic cytotoxicity increasing with the length of incubation [12]. Additionally, microglia co-cultured with N. fowleri trophozoites secreted the pro-inflammatory cytokines, TNF-α, IL-1β, and IL-6 [12]. Amebic cytotoxicity and the secretion of TNF-α from microglia were both reduced in co-culture experiments with N. fowleri by the addition of an antibody that specifically recognized Nfa1 protein, expressed on the pathogen's food-cups [12,13].
To elucidate the contact-independent mechanism for amebic pathogenicity, researchers have attempted to examine the cytopathic effects of incubating N. fowleri secreted proteins (excretory-secretory proteins; ESPs) or acanthamoebic lysate or their secreted proteins against a number of target cell types [9-11,14,15]. However, no studies have examined the specific effects of N. fowleri lysate on microglial cells in experimental models of PAM.
Rat microglial cells can differentiate into 3 distinct morphologies, including an ameboid form unique to embryogenesis, a ramified form found in the mature brain, and a rod-shaped form that congregates around inflammatory lesions in the CNS [16,17]. They can function as phagocytotic cells and additionally produce cytokines, including IL-1, IL-6, and TNF-α [18,19]. Thus, microglial cells are considered an important target cell-type in Naegleria infection of the CNS, and have consequently been extensively studied to shed light on its mechanisms of pathogenesis [9,12].
In the present study, we have utilized a combination of light microscopy and scanning (SEM) and transmission electron microscopy (TEM) to identify the morphological alterations and potential cytopathic effects exerted by N. fowleri lysate on primary cultured rat microglial cells. Additionally, we have endeavored to characterize the host cell immune responses by amebic lysate in culture by assaying microglial release of pro-inflammatory cytokines.
N. fowleri trophozoites (Cater NF69 strain, ATCC NO. 30215, Manassas, Virginia, USA) were axenically cultured at 37℃ in Nelson's medium [20]. Amebic lysate was prepared by freeze-thaw, and the soluble protein fraction assessed for degradation by SDS-PAGE electrophoresis (15% gel) as previously described [21]. Rat microglial cells were prepared using the methods of Giulian and Baker [16] and Oh et al. [12].
To observe the morphological changes induced by N. fowleri lysate, microglial cells (2×105) harvested from rats were incubated with differing concentrations of lysate (0.1, 0.5, or 1 mg/ml) adjusted to the same volume in PBS (pH 7.4) for 3, 6, or 12 hr in 24-well culture plates. PBS (100 µl) and lipopolysacharide (LPS) (1 µg/ml) incubation were used as controls. After incubation, samples were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4), post-fixed with 1% osmium tetroxide-1.5% potassium for 1 hr, and imaged with a light microscope. For electron microscopies (SEM or TEM), harvested rat microglial cells (2×105) were seeded onto Lab-Tek II chamber slides (Nunc A/S, Roskilde, Belgium), and 1 mg/ml of N. fowleri lysate was added to the culture system. After incubation for 3, 6, or 12 hr, SEM examination was carried out using the method of Oh et al. [12]. An image analyzer program (Escan 4000, Bumi-Mi Universe Co., Ltd., Ansan, Korea) was used in SEM image capture (S800, Hitachi, Tokyo, Japan). Additionally, harvested microglial cells treated with N. fowleri lysate (1 mg/ml) for 3, 6, or 12 hr were prepared for TEM via published methods [12]. After the blocks were sectioned and stained with Ultrostain 1H and 2 (Leica, Vienna, Austria), microglia were observed and imaged using a Zeiss EM 902A TEM (Leo, Oberkohen, Germany).
To quantitate the cytotoxicity of N. fowleri lysate against target microglial cells, a 51Cr (chromium) release assay was performed according to methods published previously [12]. Labeled microglia (5×104) were treated with N. fowleri lysate (0.5 or 1 mg/ml) in 5% CO2 for 3, 6, 12, or 24 hr. The percentage of radioisotope released by microglial cells was used as an index of lysis, as calculated by the following formula: Cytotoxicity (%)=(experimental release - spontaneous release)/(maximum release - spontaneous release)×100.
The amounts of TNF-α, IL-1β, and IL-6 released by lysate-treated microglial cells were analyzed according to published methods [12]. All assays used ELISA kits (BioSource International, Camarillo, California, USA) and were performed in triplicate. Data are shown as mean±SD and were statistically analyzed by the Student's t-test. Values of P<0.05 were considered significant.
N. fowleri lysate (1 mg/ml) exerted severe morphological changes, including disruption and breakdown of the plasma membrane on the rat microglia, and significantly promoted cell death (Fig. 1). These effects were dose-dependent (Fig. 1). Microglial lethality was almost complete within 6 hr culture with N. fowleri lysate, generally appearing necrotic in nature and characterized by membrane swelling and lysis of the nuclear membrane and nucleus (Fig. 2A, 2C). Some microglial cells additionally evinced hallmarks of apoptosis, such as blebbing, formation of apoptotic bodies, and nuclear chromatin condensation (Fig. 2B, 2D). In previous reports, pathogenic Acanthamoeba culbertsoni lysate induced cytopathic effects, including necrosis and apoptosis, in primary rat microglial cells [21,22], suggesting that N. fowleri contact-independent cytotoxicity proceeds similarly.
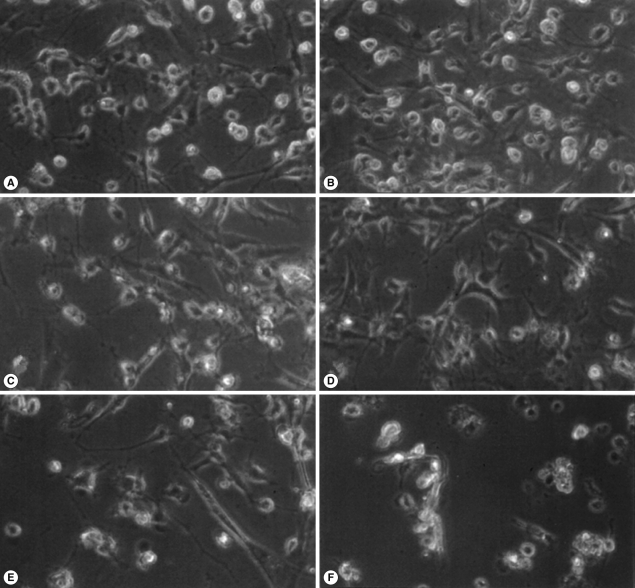
Light microscopic images of microglia treated with N. fowleri lysate for 12 hr. Microglial cells were treated with media only (A), PBS (B), LPS (1 µg/ml) (C), or different concentrations of N. fowleri lysate (0.1, 0.5 and 1 mg/ml; D-F, respectively). Microglia demonstrated significant, dose-dependent reduction in cell density. ×200.
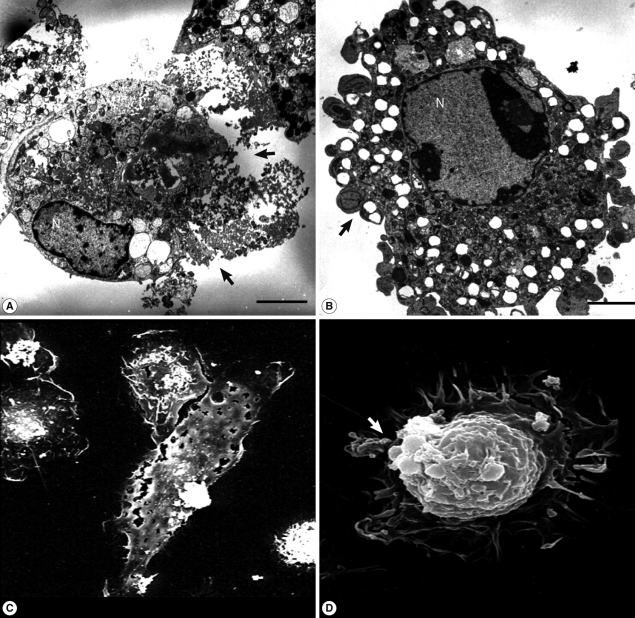
TEM and SEM images of microglia treated with N. fowleri lysate. Microglial cells were treated with N. fowleri lysate (1 mg/ml) for 12 hr. Necrosis could be observed concomitantly with plasma membrane lysis (arrows; A, C), and programmed cell death with accompanying changes, including apoptotic bodies (arrows) and nuclear chromatin condensation (B, D). Bar=5 µm.
Table 1 depicts the cytotoxic effects of treatment with N. fowleri lysate on rat microglia. Cell death increased dependent on the length of incubation, reaching 14.6, 21.9, 38.5, and 71.5% at 3, 6, 12, and 24 hr, respectively, by a 51Cr release assay. Cell death was also dose-dependent. Kim et al. [10,11] performed an LDH release assay to evaluate the cytotoxicity of N. fowleri ESPs on human microglial cells or Chinese hamster ovary (CHO) cells, and likewise found a time- and dose-dependent rise in cytotoxicity consistent with a pathogenic role for ESPs [10,11]. An analysis of N. fowleri whole-cell lysate found 2 major protein bands (13 and 21 kDa), predicted to be the Nfa1 and proteinase proteins [11,21]. These may constitute efficacious cytolytic factors in amebic lysate, and an in-depth fractionation and biochemical characterization will help elucidate the pathogenicity induced by amebic lysate.

Cytotoxicity of Naegleria fowleri lysate applied to microglial cells as assessed by 51Cr-release assay
We queried release of pro-inflammatory cytokines by microglia treated with N. fowleri lysate. TNF-α release by microglia cultured with N. fowleri lysate (1 mg/ml) was 121.6, 90.4, and 81.0 pg/ml at 3, 6, and 12 hr, respectively (Fig. 3A). IL-1β release was 88.5, 92.7, and 190.1 pg/ml, and IL-6 was 298.8, 414.9, and 251.1 pg/ml, after identical incubations (Fig. 3B, 3C). Microglial cells produce pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-3, IL-6, IL-8, IL-12, and IL-15, as a primary inflammatory immune response to host-cell damage; release of anti-inflammatory cytokines, e.g. IL-10 and TGF-β, has been reported in defense against parasitic brain infection [8,22]. We found that TNF-α and IL-6 peaked early in microglial incubation with amebic lysate, subsequently declining until the time-course's end; in contrast, IL-1β increased throughout the duration of the experiment. Microglial cells are therefore likely involved in the early inflammatory stage of N. fowleri infection through their secretion of various inflammatory cytokines, though more detailed studies will be necessary to fully illuminate the crosstalk between N. fowleri and host cells.
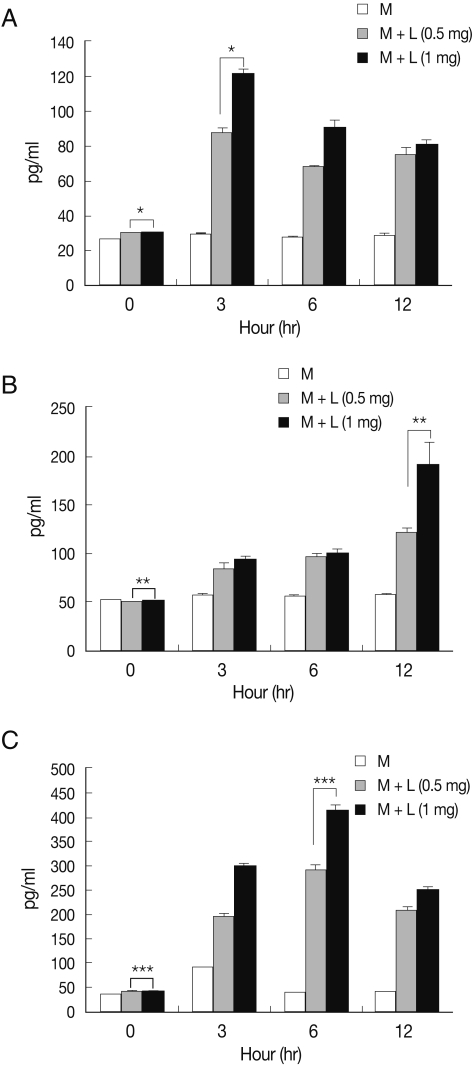
Pro-inflammatory cytokine release from microglia treated with N. fowleri lysate. Treatment of microglial cells with N. fowleri lysate for 3, 6, or 12 hr induced dose-dependent secretion of TNF-α (A), IL-1β (B), and IL-6 (C). M: microglia, L: N. fowleri lysate. *P<0.05, **P<0.05, ***P<0.05.
Acanthamoeba, comprising of several species of free-living amoebae that cause chronic granulomatous amebic encephalitis (GAE) in humans, evokes cytopathic effects on hosts through amebic adhesion to and phagocytosis of target cells, facilitated by its production of proteolytic enzymes [15,23,24], including serine proteases, contactdependent metalloproteases, elastases, cysteine proteases, and cytotoxic proteinases induced by mannose-mediated adhesion [14,25-27]. Similar contact-dependent and independent mechanisms have been proposed to contribute to N. fowleri pathogenicity [7-11]. Our findings confirm that N. fowleri lysate induces morphological cytopathic changes in microglial cells contact-independently, evoking necrotic as well as apoptotic cytotoxicity. In addition, microglia produced pro-inflammatory cytokines as a primary immune response concomitant with exposure to N. fowleri lysate.
ACKNOWLEDGEMENTS
This work (2011-0015429) was supported by Mid-career Researcher Program through NRF grant funded by the MEST.