Resistance to Toxoplasma gondii Infection in Mice Treated with Silk Protein by Enhanced Immune Responses
Article information
Abstract
This study investigated whether elevated host immune capacity can inhibit T. gondii infection. For this purpose, we used silk protein extracted from Bombyx mori cocoons as a natural supplement to augment immune capacity. After silk protein administration to BALB/c mice for 6 weeks, ratios of T lymphocytes (CD4+ and CD8+ T-cells) and splenocyte proliferative capacities in response to Con A or T. gondii lysate antigen (TLA) were increased. Of various cytokines, which regulate immune systems, Th1 cytokines, such as IFN-γ, IL-2, and IL-12, were obviously increased in splenocyte primary cell cultures. Furthermore, the survival of T. gondii (RH strain)-infected mice increased from 2 days to 5 or more days. In a state of immunosuppression induced by methylprednisolone acetate, silk protein-administered mice were resistant to reduction in T-lymphocyte (CD4+ and CD8+ T-cells) numbers and the splenocyte proliferative capacity induced by Con A or TLA with a statistical significance. Taken together, our results suggest that silk protein augments immune capacity in mice and the increased cellular immunity by silk protein administration increases host protection against acute T. gondii infection.
Silk protein consists of sericin (a glue-like protein) and fibroin (a structural protein). Silk protein is obtained from the cocoons of the mulberry silkworm, Bombyx mori. Recently, the usefulness of silk protein has been emphasized in the context of enhancing biological activity. In particular, sericin has been used as a wound dressing [1] and as an additive in cosmetics [2]. Furthermore, silk fibroin is used for matrix scaffold construction in the tissue engineering field [3]. In addition, recent research has shown that sericin is able to accelerate alcohol elimination through urine and ethanol oxidation in the liver, and the administration of silk protein hydrolysate has been shown to promote insulin release via induction of β-cell activity in pancreatic islets [5]. Accordingly, previous results suggest that silk protein can act as a biological regulator and, furthermore, mitigate disease.
T. gondii is a well-known protozoan parasite and the causative agent of toxoplasmosis. It is an opportunistic pathogen and can cause a serious disease when transmitted congenitally to immunocompromised patients [6]. In immunocompetent individuals, a chronic (latent) infection occurs [6]. The infection status of T. gondii is thus dependent on host immune capacity. In a previous study, when experimental mice were infected with more than 1×104 T. gondii tachyzoites (RH strain), the majority of the mice died within 7 days [7], whereas in another study, Th1 cytokines, including IFN-γ, TNF-α, IL-12, and IL-18, were important for the induction of effective protection against T. gondii [8]. However, very high levels of Th1 cytokines are pathogenic, whereas modest increases in Th1 cytokine levels led to minimal host tissue damage and control infection [8]. Accordingly, to elicit a protective immune response against T. gondii, modest induction of Th1-type immune responses may be important.
Some protein foods are known to contain immune-modulating agents. For example, prolonged oral administration of lactoferrin was found to restore humoral immune responses in immunocompromised mice [9] and to increase NK cell activities by increasing the productions of IL-18 and type I IFN [10]. Similarly, soybean protein fraction has been reported to stimulate innate cellular immune responses in mice [11], and casein (a milk-derived protein) has been reported to have a mitogenic effect on spleen cells [12]. These reports demonstrate that an oral ingestion of certain proteins can change immunity, and thus influence resistance to a disease. Recently, silk protein, which consists of polypeptides, was proposed as a bioactive supplement [1]. In this study, we examined the effects of silk protein on immune responses in mice infected with T. gondii. Furthermore, to determine the effects of silk protein on innate immune capacity, we also investigated whether silk protein can overcome the immune suppression induced by methylprednisolone in mice.
Hydrolyzed silk protein was prepared from the cocoons of the silk worm, B. mori (World Way Co., Ltd., Yeongi, Chungcheongnam-do, Korea). Briefly, 90 g of cocoons were cut into small pieces and boiled in 1 L of 50% CaCl2 for 6 hr at 111℃±5℃ and then filtered through a filter paper. The solution was clarified using an ion exchange membrane electrodialyzer (Asahi Kasei Co, Tokyo, Japan) [5], and then freeze-dried using a Benchtop Freeze Dry System (Labconco Cop., Kansas City, Kansas, USA) before experiments. The silk protein obtained was administered to mice at dilutions of 200 or 500 mg/kg/day for 6 consecutive weeks. The composition of the freeze-dried silk protein was analyzed at the Korea Food Research Institute (Gyeonggi-Do, Korea), and found to be composed of almost 100% protein (data not shown). To determine whether or not the silk protein used was contaminated with bacterial endotoxin, a LAL Endotoxin Assay Kit (GenScript USA Inc., Piscataway, New Jersey, USA) was used to measure endotoxin levels, which were found to be 0.25 EU/ml in the freeze-dried protein, lower than its level in drinking water [13].
Specific pathogen-free (SPF) male BALB/c mice were purchased from DooYeol Biotech (Seoul, Korea). After stabilization for 1 week, mice were housed in an animal facility under SPF conditions at Seoul National University College of Medicine. All experiments were conducted in accordance with the guidelines issued by the Institute of Laboratory Animal Resources at Seoul National University (license number: SNU-090424-3). Seven-week-old male BALB/c mice were administered silk protein or PBS (control) using a gavage needle (Cadence Science, Lake Success, New York, USA) daily for 6 weeks. Each experimental group consisted of 5 mice. Mice were administered PBS (n=20) or 500 mg/kg of silk protein (n=20) for 6 weeks and then were infected with 1×107 tachyzoites of T. gondii (RH strain) orally [14]. Mice in immune suppression groups administered PBS (normal control group; n=4) for 6 weeks, PBS with injection of 20 mg/kg of methylprednisolone acetate (methisol; an aqueous suspension containing methylprednisolone acetate at 40 mg/ml [Shinpoong Pharm Co., Seoul, Korea]) (the immunosuppression control group; n=6), or 200 mg/kg of silk protein with injection of 20 mg/kg of methylprednisolone acetate (methisol) (the immunosuppression experimental group; n=7) into the muscles to induce immune suppression at last 2 weeks during silk protein administration for 6 weeks. The optimal dose of methylprednisolone acetate per mouse was determined beforehand by preliminary experimentation to induce immune suppression without causing obvious adverse effects. To determine resistance against T. gondii infection, T. gondii tachyzoites were prepared by serial passage in mice [15] and orally inoculated into experimental mice. T. gondii lysate antigen (TLA) was prepared as previously described [16]. The protein concentration of membrane-filtered TLA was determined using BCA™ Protein Assay Kits (Thermo Scientific, Rockford, Missouri, USA), and the antigen was stored at -70℃ until required.
For in vitro experiments, the spleens of silk protein-administered mice were placed in sterile dishes under complete RPMI-1640 media (WelGENE Inc., Daegu, Korea) containing 10% FBS (Invitrogen, Carlsbad, California, USA), 10 mM HEPES, 2 mM L-glutamine, 2 mM sodium bicarbonate, 5×10-5 M 2-mercaptoethanol, and antibiotics (100 IU/ml penicillin G and 100 µg/ml streptomycin; Invitrogen) and used to prepare single cells, as previously described [17]. Spleen erythrocytes were destroyed by hypotonic shock using a red blood cell lysis buffer containing NH4Cl, and after washing, splenocytes (1×106) were subjected to fluorescence-activated cell sorter (FACS) analysis [17]. The phenotypic ratios of CD4+-T cells, CD8+-T cells, macrophages, and natural killer cells were examined using a BD FACS Flow cytometer (BD Biosciences, Mountain View, California, USA). Non-specific binding of fluorescein-conjugated rat monoclonal antibody was inhibited by treating with rat anti-mouse CD16/32 mAb (eBiosciences, San Diego, California, USA). The specific antibodies used for FACS analysis were PE-conjugated anti-mouse CD4 (L3T4; eBiosciences), PE-Cy5-conjugated anti-mouse-CD8a (Ly-2; eBiosciences), FITC-conjugated pan-NK cells (CD49b; eBiosciences), and PE-conjugated anti-mouse F4/80 (eBiosciences). The fluorescences of labeled cells were quantified using a BD FACS Flow Cytometer (BD Bioscience). Data are expressed as percentages of 1×104 spleen cells. Splenocytes proliferation (5×105) after exposure of Con A (5 µg/ml; Merck, Frankfurter Str., Germany) or T. gondii lysate antigen (TLA) (100 µg/ml) was determined using the MTT assay (Cell Viability Assay Kit, Daeil Lab Service Co., Seoul, Korea). Cellular proliferation was measured at 490 nm using an Emax Precision microplate reader (Molecular Devices, Sunnyvale, California, USA). Cytokine assays were performed as follows. Splenocytes (5×105) of PBS- and silk protein-administered mice were seeded in 96-well culture plates with 200 µl of complete RPMI-1640 media containing 10% FBS and cultured at 37℃ for 48 hr in a 5% CO2 incubator. Supernatants of experimental groups were pooled and analyzed for cytokine levels using a mouse cytokine array kit (RayBiotech, Inc., Norcross, Georgia, USA). Signal intensities were quantified using a densitometer (Multi Gauge, Fuji Film, Tokyo, Japan) and images were processed using a luminescent image analyzer (Fuji Film). Statistical analysis was performed using the unpaired Student's t-test. P values of <0.05 were regarded as statistically significant, and results are presented as the mean±SD.
The immune responses and survival of T. gondii-infected mice were examined after administering silk protein. The administration of 500 mg/kg of silk protein induced a significant increase in CD4+ and CD8+ T-cells among splenocytes (P<0.05), whereas populations of NK cells and macrophages were unchanged (Fig. 1A). T cell-derived cytokines, i.e., Th1 and Th2 cytokines in 2 broad classes for effector CD4+ Th cells, were obviously increased, and in particular, IFN-γ increased by 3.0-fold (Fig. 1B). The signal intensity of cytokines representing as an increased fold value of experimental group compared to control group was as follows: Th1-related cytokine increases were TNF-α (1.2-fold), IL-2 (1.4-fold), IL-12 (1.6-fold), and IFN-γ (3.0-fold), and Th2-related cytokine increases were IL-10 (1.4-fold), IL-5 (1.5-fold), IL-13 (1.6-fold), and IL-4 (1.7-fold) (Fig. 1B). Accordingly, our data showed that the administration of silk protein strongly induced IFN-γ (3.0-fold) as compared with other cytokines (Fig. 1B). This result suggests that the administration of silk protein induces T-cell immune response involving IFN-γ induction, and that this immune response contributes to host protection against T. gondii infection (Fig. 1D).
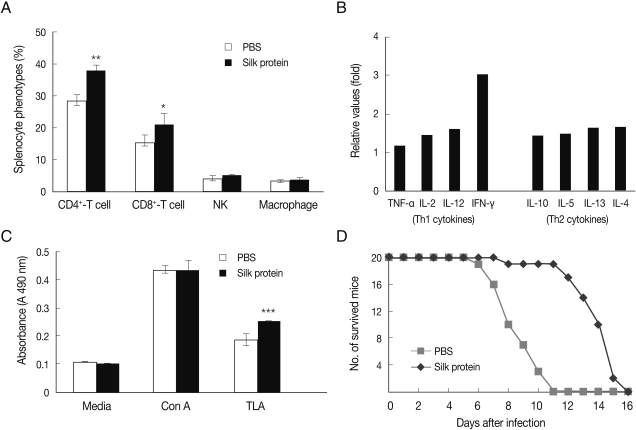
Characteristics of immune responses in mice administered with silk protein. (A) Ratios of CD4+ T-cells, CD8+ T-cells, natural killer cells, and macrophages in the spleens of mice administered with PBS or silk protein for 6 weeks. Phenotypes of splenocytes were examined by FACS. (B) Profiles of cytokines secreted by the spleen cells of mice administered with silk protein. Splenocytes were cultured for 3 days without further stimulation and cytokine levels in the supernatants were examined. Data are expressed as fold changes in cytokines in the silk protein-administered group versus PBS treated control group. (C) Proliferation of spleen cells in mice administered silk protein for 6 weeks. Splenocytes were cultured with Con A or T. gondii antigen (TLA), and the proliferation was determined by MTT assay. When splenocytes were stimulated with TLA, proliferative capacities significantly increased in mice administered with silk protein. (D) Survival of mice administered with PBS or silk protein after an oral inoculation of 1×107 T. gondii (RH strain) tachyzoites. Data are means±SDs. P-values indicate significant differences between PBS- and silk protein-administered mice (*P<0.05, **P<0.005, ***P<0.0005).
When mice were inoculated orally with 1×107 T. gondii tachyzoites, the survival time of silk protein-administered mice was longer than that of the PBS treated mice (Fig. 1D). This experiment was performed using 20 mice per group. Furthermore, a half (n=10) of 20 mice of PBS group died within 8 days of infection and all died within 11 days. In contrast, a half (n=10) of 20 mice administered with silk protein survived until 14 days after infection, though all mice succumbed by 16 days after infection. These results were correlated with the increased splenocyte proliferation observed after stimulation with TLA (Fig. 1C; P<0.005).
To verify the effects of silk protein on immune responses, we compared degrees of immune suppression after injection with methisol solution containing methylprednisolone acetate in PBS- and silk protein-administered mice. Methisol administration to control mice resulted in a marked decrease in the total spleen cell number (PBS group; 7.91±0.39×107, PBS+methisol group; 3.83±0.37×107, P<0.05; Fig. 2A). However, silk protein weakened the degree of immune suppression (silk pro-tein+methisol group; 6.76±0.25×107) as compared with PBS treated mice (PBS+methisol group; 3.83±0.37×107) (P<0.005; Fig. 2A).

Characteristics of immune responses after treatment with methylprednisolone acetate in PBS- and silk protein-administered mice. Total numbers of spleen cells (A), phenotypes of splenocytes (B), and proliferative capacities of splenocytes in response to Con A and TLA (C) were compared in the PBS, PBS+methisol, and silk protein+methisol groups. Cell phenotypes of CD4+ and CD8+ T-cells were examined by FACS and splenocyte proliferation was measured using the MTT assay. Data are means±SDs. P-values indicate significant differences between the PBS, PBS+methisol, and silk protein+methisol groups. (*P<0.05, **P<0.005).
Splenic CD4+ T-cell populations (%) in the PBS, PBS+meth-isol, and silk protein+methisol groups were 28.6±1.8, 13.1±0.6, and 22.7±0.8, respectively (Fig. 2B). Similarly, ratios (%) of CD8+ T-cells in the respective groups were 15.9±2.6, 8.1±0.6, and 12.7±0.8 (Fig. 1B). Mice administered with silk protein (silk protein+methisol group) were found to be protected against immune suppression. Methisol also dramatically decreased the proliferation of splenocytes induced by mitogen (Con A) stimulation (Fig. 2C). In this case, the administration of silk protein also inhibited the suppression of cell proliferation suggesting that it has a mitogenic effect (Fig. 2C). Recently, silk protein was reported to mitigate liver damage caused by alcohol, which suggests that it enhances ethanol oxidation rates [4]. Besides, silk protein was reported to be used as a biological active supplement regulating blood glucose [5]. However, it has not been examined whether the disease modifying effect of silk protein is related to in vivo immune enhancement. Moreover, it remains to be elucidated whether the host resistance against T. gondii is related to innate immune capacity enhancement by silk protein.
According to our results, the administration of silk protein resulted in increases of splenic CD4+ and CD8+ T-cells and proliferative capacities of splenocytes. It has been well-established that CD4+ and CD8+ T-cells play a major role in cellular immune responses and that they are important components of host defense against infections [19,20]. In addition, our results showed that T-cells in splenocytes of mice administered with silk protein proliferated in response to in vitro stimulation with TLA (P<0.005). Similarly, the administration of silk protein increased the survival of mice. Accordingly, it is suggested that the increased resistance against T. gondii infection is correlated with host immune capacity.
Host defense against parasite infections is usually attributable to acquired immunity, which is related to T-lymphocytes. Protective immunity against T. gondii infection is induced by cell-mediated immune responses and productions of Th1 cytokines, such as IFN-γ and TNF-α [8,14,18]. Because the administration of silk protein was found to increase T-cell numbers and the expressions of T-cell-mediated cytokines, especially IFN-γ, it could be expected that silk protein possess mitogenic characteristics and induces non-specific T-cell proliferation as do other protein supplements, like lactoferrin [9] and soybean protein [21].
Several bacterial components, including lipopolysaccharides (LPS), liposomes, and DNA, are the known immunomodulators [22]. Recently, some food components were found to have immunomodulatory effects [11]. Casein and soybean are the known immunomodulators [11,21]. In a previous report, a casein phosphopeptide preparation was found to stimulate mucosal IgA responses in mice [23], and in another lactoferrin was found to increase NK cell activities by increasing the productions of IL-18 and type I IFN in the mouse small intestine [10]. From this perspective, it appears that silk protein is an oral immunomodulator. To confirm the effects of silk protein on T-cell proliferation, we assessed its effects on the immune suppression induced by methylprednisolone treatment. The administration of silk protein was found to inhibit decreases significantly in numbers of spleen cells, CD4+ and CD8+ T-cell ratios, and proliferative responses of splenocytes induced by methylprednisolone (Fig. 2). Methylprednisolone acetate is usually used to create immune suppression models [24], and the mechanism involved is known to be its anti-proliferative and/or apoptotic effects on cells [25]. In a previous study, mice injected with methylprednisolone acetate showed a decrease in circulating CD4+ T-lymphocytes, and enabled the propagation of Cryptosporidium parvum in adult mice [24]. Accordingly, because the effects of methylprednisolone are related to a decrease in T-lymphocyte numbers [24,26,27], it is possible that the proliferation of CD4+ and CD8+ T-cells induced by silk protein confers resistance to the immunosuppressive state induced by methylprednisolone.
Taken together, the present study showed that silk protein confers host protection against T. gondii infection by increasing T-cell proliferation and production of Th1 cytokines, such as IFN-γ. Our results suggest that silk protein should be regarded as a potential immune enhancing food supplement.
ACKNOELEDGEMENTS
This work was supported by Worldway Co. Ltd, South Korea (grant nos. 20080822 & 20090424).