Partial Purification and Characterization of a Cysteine Protease Inhibitor from the Plerocercoid of Spirometra erinacei
Article information
Abstract
Helminthic cysteine proteases are well known to play critical roles in tissue invasion, nutrient uptake, and immune evasion of the parasites. In the same manner, the sparganum, the plerocercoid of Spirometra mansoni, is also known to secrete a large amount of cysteine proteases. However, cysteine protease inhibitors regulating the proteolytic activities of the cysteine protease are poorly illustrated. In this regard, we partially purified an endogenous cysteine protease inhibitor from spargana and characterized its biochemical properties. The cysteine protease inhibitor was purified by sequential chromatographies using Resource Q anion exchanger and Superdex 200 HR gel filtration from crude extracts of spargana. The molecular weight of the purified protein was estimated to be about 11 kD on SDS-PAGE. It was able to inhibit papain and 27 kDa cysteine protease of spargana with the ratio of 25.7% and 49.1%, respectively, while did not inhibit chymotrypsin. This finding suggests that the cysteine protease inhibitor of spargana may be involved in regulation of endogenous cysteine proteases of the parasite, rather than interact with cysteine proteases from their hosts.
Human sparganosis is a disease caused by invasion of the sparganum, the plerocercoid of Spirometra mansoni. Human infections are caused by drinking of freshwater contaminated with cyclops infected with procercoids or ingestion of raw materials of plerocercoid larvae in intermediate hosts such as frogs and snakes [1]. Considering the penetration ability of spargana, the importance of proteases of the parasite has been well documented [2-5]. Helminthic parasite's proteases have been known to play major roles in tissue penetration, nutrient uptake and immune evasion of the parasite [6]. A purified 27 kDa cysteine protease (CP) in spargana has been proven to be important in tissue penetration and immune evasion of the parasite by cleaving host's immunoglobulin G (IgG) [4]. The roles of the sparganum CP are well documented in many studies [2-5], however, cysteine protease inhibitor (CPI) as a modulator of the proteolytic activity of proteases is not reported elsewhere.
Several studies on the protease inhibitors of parasites have been illustrated. For example, a 43/41 kDa serine protease inhibitor of Toxoplasma gondii showed inhibitory activities on chymotrypsin and trypsin [7]. In addition, cystatin, a kind of CPI, has been documented in some parasites [8-11]. The present study aimed to identify CPI and partially purify endogenous CPI of spargana. Moreover, the biochemical properties of purified CPI were also partially characterized.
Crude extracts of spargana were prepared as described previously [4]. Briefly, spargana were collected from naturally infected snakes and the parasites were washed several times with sterile saline and homogenized in a Teflon pestle homogenizer with Tris-HCl buffer (0.01 M, pH 7.4) containing 0.1 M NaCl. After centrifugation at 15,000 rpm for 30 min at 4℃, the resulting supernatant was used as crude extracts.
For the purification of CPI, gel filtration and ion exchange chromatography were performed with some modifications [14]. The crude extracts of spargana were loaded onto a Superdex 200 HR gel filtration column using ACTA FPLC system (Amersham pharmacia Biotech, Piscataway, New Jersey, USA). Each column fraction was collected and monitored for CPI as follows. Papain (Sigma, St. Louis, Missouri, USA) and the endogenous sparganum CP which was purified from spargana as described previously [4] were incubated with an equal volume of crude extracts or each column fraction at room temperature for 20 min. Then, fluorogenic synthetic substrate, carbobenzoyl-phenylalanyl-arginyl-7-amino-4-methylcoumarin (Z-FR-AMC; Sigma, St. Louis, Missouri, USA) in the presence of 2 mM DTT was added [12,13], and the reaction mixtures were further incubated for 30 min at 37℃ and the released AMC was measured. The fractions which showed inhibitory effects on the proteolytic activity were collected. They were dialyzed against Tris-HCl buffer (10 mM, pH 7.4) and then loaded onto a Resource Q anion exchanger previously equilibrated with the same buffer. The column was washed with the same buffer and eluted with NaCl by increasing molarity up to 1 M. The activity of CPI was detected in each column fraction as described above, and active fractions were pooled and monitored their purity by SDS-PAGE.
An inhibition assay of the purified CPI was performed using papain and partially purified endogenous 27 kDa CP of spargana with the same method described above. Also, gelatin (final 0.2%, v/v) containing SDS-PAGE was done under non-reducing condition with or without the partially purified CPI by the method of Kim et al. [14].
When crude extracts of spargana were loaded onto Superdex 200 HR gel filtration, 8 protein peaks were observed. Of them, the 27 kDa CP was monitored at fractions 33 to 38 and CPI activity was detected at the peak fraction around 42 to 44 (Fig. 1A). The fractions of CPI were pooled and dialyzed against Tris-HCl buffer (0.01 M, pH 7.4) and loaded onto a Resource Q anion exchanger column for further purification. CPI was eluted by a high concentration of NaCl, thereby, CPI was presumed to bind tightly on the column (Fig. 1B). The fraction 42 was selected and used as a partially purified CPI for further studies.
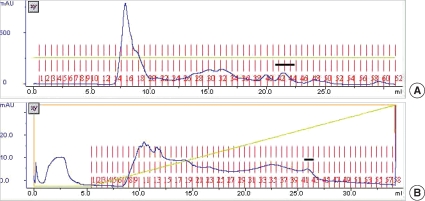
(A) Elution profiles of Superdex 200 HR gel filtration chromatography. The column was eluted with Tris-HCl buffer (0.01 M, pH 7.4) containing 0.1 M NaCl at a flow rate of 0.3 ml/min. Cysteine protease inhibitor activity (CPI) fractions are indicated as bar. The active fractions were pooled and concentrated. (B) Elution profiles of 2nd Resource Q anion exchanger chromatography. The column was eluted with Tris-HCl buffer (0.01 M, pH 7.4) increasing molarity of NaCl. The flow rate was 1 ml/min, and it was collected 0.5 ml/tube. The CPI fractions are indicated as bars.
As shown in Fig. 2, the partially purified CPI migrated at 11 kDa on SDS-PAGE. Cystatin, one of the widely known CPIs, is divided into 3 major groups; 11 kDa stefin without disulfide bridge, 14 kDa cystatin with disulfide bridge, and 60-120 kDa glycoprotein kininogen [15]. Although molecular sequencing of the purified CPI is not accomplished, it is likely that the purified CPI belongs to the stefin group simply by comparison of molecular masses. Molecular cloning of the CPI would be necessary to confirm the biochemical nature of the CPI on-going studies.
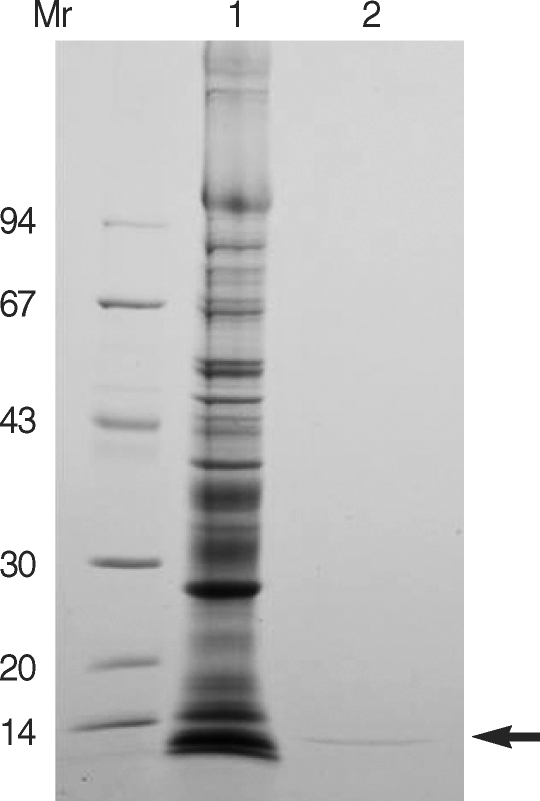
SDS-PAGE findings of purified cysteine protease inhibitor (CPI) from the sparganum crude extracts. The gel was analyzed on 7.5-15% gradient gel. M, standard marker proteins; lane 1, crude extracts of spargana; lane 2, partial purified CPI of Resource Q column fraction. Arrow indicates the purified CPI.
In the inhibition studies, cystatin could inhibit papain and the sparganum CP with the ratio of 78.4 and 79.1%, while the purified CPI could inhibit only 22 and 49%, respectively (Table 1). In addition, both inhibitors did not significantly affect the activity of chymotrypsin, one of the typically known serine proteases. These results suggest that the CPI is a constituent protein of spagana and CPI shows an inhibitory ability reacting effectively against the endogenous 27 kDa spaganum CP than papain.
The gelatin incorporating SDS-PAGE study also showed that the purified CPI could inhibit endogenous 27 kDa CP of spargana (Fig. 3). Degrading gelatin by the 27 kDa CP was decreased by increasing the CPI amount (Fig. 3, lane 3). The pattern of the gelatin gel analysis was almost identical to that of results obtained with the inhibition study. This result also demonstrates that the purified 11 kDa of spargana was probed to be a cysteine protease inhibitor.
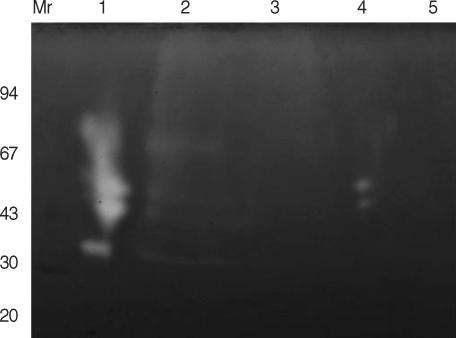
Gelatin containing SDS-PAGE findings of purified cysteine protease inhibitor (CPI) under non-reducing condition. The gel was analyzed on 7.5-15% gradient gel. M, standard marker proteins; lane 1, partially purified 27 kDa cysteine protease (CP) without inhibitor; lane 2, 27 kDa CP with CPI (5 µg); lane 3, 27 kDa CP with CPI (10 µg); lane 4, 27 kDa CP with cystatin (10 µg); lane 5, 27 kDa CP with iodoacetic acid (IAA, 20 µM). Proteolysis was shown on white area.
Cystatin is a wide spread protein in mammals and parasites with several functional roles including regulation of proteases [9,15,16]. We attempted primarily to detect and characterize the CPI from the spargana. However, the limited amount of the purified CPI led us to only a partial characterization of the CPI. Although the precise roles of the CPI are unclear in this study, it is presumed that the sparganum CPI may be involved in the regulation of endogenous CPs other than interaction with CPs from their host. In this way, it is necessary to study the interactions between action mechanisms and physiological roles of CPI against CP, and finally molecular cloning of CPI in spargana should be performed.
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Grant of Ewha Womans Universty.
