Production and Evaluation of Toxoplasma gondii Recombinant GRA7 for Serodiagnosis of Human Infections
Article information
Abstract
The precise diagnosis of the acute toxoplasmosis in pregnant women and immunocompromsied patients has critical importance. Most of the commercially available assays use the whole Toxoplasma soluble extract as the antigen. However, the assays currently available for the detection of specific anti-Toxoplasma antibodies may vary in their abilities to detect serum immunoglobulins, due to the lack of a purified standardized antigen. The aim of this study was production and evaluation of the usefulness of the recombinant Toxoplasma gondii GRA7 antigen for the serodiagnosis of Toxoplasma gondii IgM and IgG by ELISA. A total of 70 T. gondii IgM positive sera, 74 T. gondii IgG positive sera, and 60 sera from subjects who were not infected with T. gondii were examined. These sera were shown different absorbance values in ELISA test. To control the specificity of the rGRA7 other parasitic diseases, for example, echinococcosis, malaria, leishmaniasis, fascioliasis, and strongyloidiasis were tested of which none showed positive results. Sensitivity and specificity of the generated recombinant IgG ELISA in comparison with commercial ELISA (com ELISA) were 89% and 90%, and the sensitivity and specificity of the generated recombinant IgM ELISA were 96% and 90%, respectively. The results obtained here show that this antigen is useful for diagnostic purposes.
Most infections with Toxoplasma gondii in humans are asymptomatic although first exposure to the parasite during pregnancy may cause abortion or congenital malformation. The disease is often fatal for immune suppressed patients such as those with acquired immunodeficiency syndrome [1]. The tests presently used for toxoplasmosis diagnosis are based on serological assays. Although they give satisfying results, accurate differentiation between recently acquired and chronic toxoplasmosis remains problematic. False positive reactions with antinuclear antibodies, rheumatoid factors, or naturally occurring human antibodies and false negative reactivity due to competitive inhibition by high levels of specific IgG antibodies have been described [2]. The presence of specific IgM antibodies is not always indicative of an acute infection with T. gondii, because in some cases IgM antibodies can persist for months or years [3].
The usefulness of tests for diagnosis of toxoplasmosis has been limited by the inability to obtain standardized reagents. This is due to the fact that T. gondii is obligatory intracellular parasite therefore, antigens always contaminated with the host cell, various non parasitic materials from culture media in which the parasite is grown. The methods of producing tachyzoites as well as antigen(s) may also vary significantly between laboratories [4]. Therefore, as soon as DNA technology became available for the production of recombinant antigens, they were considered to have the ability to replace natural antigen (s) obtained from lysed whole parasites. The major advantages of recombinant antigens for the diagnosis of T. gondii infections are (1) the antigen composition of the test is precisely known and caused less false positive and false negative (2) more than one defined antigen can be used and (3) the method can be easily standardized [5].
Dense granule antigens (GRA), secreted in abundance, are major components of both the vacuole surrounding tachyzoites and cyst wall surrounding slower-growing bradyzoites[6]. The dense granules have an essential role in the cell invasion, maintenance of the parasitophorous vacuole, and survival of the parasite after cellular invasion [6]. In almost all nucleated host cells GRA proteins are potent antigens that produce strong T and Bcell responses during infection. Immunological responses to GRA7 may be important in controlling infection, as immunization with the native protein partially protects mice against acute toxoplasmosis [7]. While granule protein 7 produces very strong antibody response in the acute phase of infection , mutant parasites lacking GRA7 exhibit slow growth and pronounced morphological defects when cultured under nutrient-limiting condition [6,7].
In this study, the recombinant protein of dense granule antigen GRA7 of T. gondii was used for the recognition of acute and chronic phase of toxoplasmosis in human sera [8,9]. The tachyzoites of T. gondii RH strain were inoculated to the peritoneal cavity of BALB/c mice. After 3 days, the parasites were collected, washed, and resuspended in PBS (pH 7.2). Genomic DNA of T. gondii RH strain was isolated by the conventional phenol, chloroform, and ethanol precipitation method. Genomic DNA isolated from tachyzoites was used as a template to amplify the GRA7 gene by PCR reaction. A pair of primer based on GRA7 gene sequence was designed with Bam H1 and Not1 restriction sites. (GRA Forward: 5'-GGATCCATGGCCCGACACGCAAT-3'), (GRA Reverse: 5'-GCGGCCGCCTGGCGGGCATCCTC-3'). PCR reaction was performed in a total volume of 50 µl using 50 ng DNA, 1.5 µl forward and reverses primers at 10 pmol, 50 mM Mgcl2, 200 mM dNTP, 10x PCR buffer, 2.5 unit Taq polymerase. PCR reaction was carried out with 30 cycles of denaturation at 94℃ for 40 sec, annealing at 58℃ for 60 sec, and extension at 72℃ for 60 sec. Reaction was incubated at 94℃ for 5 min before beginning the PCR cycle, and ended with a final extension at 72℃ for 10 min in a thermal cycler. The amplified DNA of GRA7 gene was visualized on 1% agarose gel stained with syber green. Then, the DNA band was cut and recovered by the DNA purification kit (Fermentas, Berlin, Germany).
The recovered DNA was cloned into the PTZ57R cloning vector (Fermentas, Berlin, Germany) via T/A PCR product cloning kit (Fermentas) according to the manufacturer's protocol. The ligation reaction was transformed in E. coli XL1-blue strain competent cells and dispensed on agar plate containing 100 mg/ml ampicillin. Bacterial colonies were screened by agar plate containing X-gal (Fermentas) and isopropyl-D-thiogalactopyranoside (IPTG) to discriminate between recombinant (white) and non-recombinant (blue) containing colonies [10]. The recombinant plasmid was detected by restriction analysis with BamH1 and Not1 enzymes [11], and the GRA7 fragment was extracted from 1% agarose gel by DNA purification kit (Fermentas).
The GRA7 gene was subcloned in the pET-28a expression vector. Reaction was transformed in E. coli Top10F with Kanamycine and colonies contained recombinant plasmids were mass cultured on LB medium. The plasmid with the correct insert was confirmed by restriction enzymes, PCR analysis and sequencing method. Escherichia coli strain Top10F containing pET28a-GRA7 was grown with vigorous shaking (250 rpm) at 37℃ in liquid broth (LB) with Kanamycine to an optical density at OD 0.600. Protein production was then induced with 1 mM (IPTG) and the cells incubated with shaking at 37℃ for an additional 4 hr.
SDS-PAGE with 12% acryl amide gel was performed. E. coli-GRA7 without IPTG and E. coli-GRA7 with IPTG was compared and then induced band was surveyed in comparison with uninduced band. In SDS-PAGE analysis rGRA7 was found to resolve at 29 kDa after induction (Fig. 1A). The gels were either stained with Coomassie blue or were used for western blots. For western blots, proteins were transferred on to nitrocellulose membranes (Biotech). After transfer, the nitrocellulose membrane was blocked in a blocking solution (Skimmed milk) for 1 hr at room temperature. After washing, strips of nitrocellulose membrane were incubated with positive T. gondii human sera diluted 1:50 in skimmed milk. After washing, strips were incubated for 1 hr at room temperature with rabbit anti-human IgM conjugate (diluted 1:500 in skimmed milk). After adding substrate, the reaction was stopped by washing in distilled water. The western blot result is presented in Fig. 2, which showed a reaction against antigen of 29 kDa for GRA7.
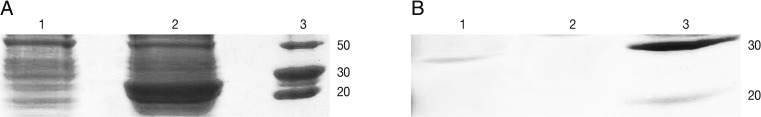
SDS-PAGE analysis of rGRA7 expression using 12% acrylamide gel. (A) Lane 1, uninduced culture; Lane 2, expression after 7 hr of induction; Lane 3, molecular protein marker. (B) Lane 1, purified rGRA7 protein (29 kDa); Lane 3, molecular protein marker.

Western blot analysis of the rGRA7 protein. Recombinant GRA7 protein was detected using a positive Toxoplasma gondii human sera and rabbit anti-human IgM conjugate by immunoblotting. Lane 1, induced control culture of cells lacking the GRA7 insert; Lane 2, purified rGRA7protein (29 kDa).
Purification procedure by Ni-NTA purification system (Invitrogen, New York, USA) was carried out according to the manufacturer's protocol. For this, 8 ml of lysate was prepared under native conditions and added to a prepared purification column. Settled the resin by gravity and carefully aspirated the supernatant and saved for SDS-PAGE analysis, washed with 8 ml native wash buffer (pH=8) again settled the resin by gravity and carefully aspirated the supernatant and saved for SDS-PAGE analysis. Around 6 mg of His-tag-GRA7 was purified from 100 ml of induced culture (Fig. 1B).
The protein concentration was determined by the Bradford method [12], and used in ELISA test. Total 204 serum samples were collected from different laboratories in Tehran. This study was reviewed by the Ethical Committee of Tehran University of Medical Sciences, as well as written informed consent was obtained from each participant). Among them, 30 had clinical symptoms, for example, fever and lymphadenopathy. In advance, the sera were tested by the Toxoplasma IgG ELISA kit (Trinity, New York, USA) and Toxoplasma IgM ELISA kit (Trinity). The tested sera were divided into the following groups: (a) 74 T. gondii IgG positive samples, (b) 70 T. gondii IgM positives (36 sera were IgG and IgM both positive), and (c) 30 sera who had no serological evidence of toxoplasmosis. We also included sera from 14 patients infected with malaria, 5 infected with Echinococcus granulosus, 4 infected with Fasciola hepatica, 1 infected with Strongyloides, 5 infected with Leishmania, and 1 infected with hepatitis B virus for checking the recombinant antigen for cross-reactivity with heterologous antibodies. All of these sera were negative for IgM and IgG Toxoplasma antibodies. Sera that contained IgM antibodies against Toxoplasma considered as acute and sera that contained IgG antibodies were considered as chronic toxoplasmosis. However, IgM antibody to T. gondii may be detectable for as early as 2 months in some individuals and for more than 1 year in others [13].
The optimal working dilution of recombinant antigen and conjugate was determined by checkerboard assays using serial dilutions of antigen, sera and conjugate. The checkerboard assay with rGRA7, determined a working dilution of 7.5 µg/ml recombinant antigen per well for IgM positive sera and 5 µg/ml for IgG positive sera. For determination of the optimal serum dilution, sera were diluted from 1:10 to 1:1,280. The dilution that showed the highest difference in optical densities (OD) between positive and negative sera was selected for screening of all the sera. A serum dilution of 1:20 for acute toxoplasmosis and 1:100 for IgG positive sera were selected for the screening of single sera as this dilution revealed the highest difference in OD values between positive and negative sera. The sera were tested duplicate and the mean absorbance value was calculated. OD values obtained with serial dilutions of the positive and negative sera under the optimal assay conditions.
Purified recombinant antigen was individually diluted to the optimized concentration of 5-7/5 µg per ml in bicarbonate buffer (pH=8) and 0.1 ml of each antigen was added to separate wells of micro titer plates. Blank well, negative control and positive control were considered. Plates were washed with PBST, blocked with blocking buffer. Serums were diluted (1:100) for IgG antibody ELISA and (1:20) for IgM antibody surveys. After adding of diluted sera, plates were incubated then washed with PBST. Bound human IgG was detected by adding anti-human IgG conjugated with horseradish peroxidase (Dako, Skagen, Denmark). Human IgM was detected by using anti-human IgM conjugated with horse radish peroxidase as well.After incubation the plates (Nunc, Skagen, Denmark) were washed then the chromogenic substrate orthophenylenediamine (Merck, Berlin, Germany) was added. The reaction was stopped by adding 1 M sulfuric acid and the optical density was read by ELISA reader (Lab System, Helsinki, Finland) at 492 nm.
Sensitivity and specificity obtained from TP/TP+FN *100 and TN/TN+FP *100 formula, respectively. Positive predictive value and negative predictive value obtained from TP/TP+FP and TN/TN+FN formula, respectively. All the data were analyzed by chi-square at confidence level of 95% by SPSS version 11.5. The sensitivity and specificity of this test using sera from patient with IgM positive serum was 96% and 90%, respectively. The sensitivity and specificity of this test using sera from patient with IgG positive serum was 89% and 90%, respectively (Table 1). Optical density of anti-Toxoplasma IgM and IgG by ELISA was shown in Table 2.

The sensitivity and specificity of rGRA7-IgM or IgG in comparison with results from commercial IgM and IgG ELISA kit
Routine diagnostics of T. gondii infection sometimes are unsatisfactory. A precise distinction between acute and latent toxoplasmosis may be difficult because IgM may be present in sera for many years [14]. This problem necessitates development of an alternative and more reliable diagnostic approach using recombinant antigens [15]. In this study, for acute phase sera the sensitivity and specificity rGRA7 was 96% and 90%, respectively. For chronic phase sensitivity and specificity was 89% and 90%, respectively, that it is higher than sensitivity in former studies [16].
Recombinant proteins for Toxoplasma serodiagnosis previously has been applied by many researchers. rGRA7 antigenicity has been checked using western blot analysis [17]. They mentioned human sera with acute toxoplasmosis strongly reacted with rGRA7 that confirm our result and showed rGRA7 is the most important recombinant protein for detection acute toxoplasmosis. Nigro et al. [18] showed similar sensitivity values for rSAG1and rROP2 for both chronic and recently infected groups of animals suggesting that these recombinant proteins are not useful as serological markers to discriminate between these 2 infected groups. Li et al. [19] investigated efficacy of GRA7-ELISA for distinguishing between acute and chronic toxoplasma infection, which applied GRA7 in IgG ELISA test for discriminating 10 acute sera from 10 chronic sera and reported sensitivity and specificity of 80% and 90% respectively. They cloned GRA7 gene in pMAL-C2 and transformed GRA7-Pmal into E. coli strain JM101. It seems that the number of samples is not enough for deduction. In addition, this fusion protein (GRA7 with MBP tag) is very large and it could make cross reaction in ELIZA tests.
The other study applied GRA7 in IgM ELISA for detection of acute toxoplasma infection and reported sensitivity values 50% that was lower than our sensitivity [20]. They used from pUC8 or pDS1vector and E.coli JM109. This difference could be due to the method of purification and blotting which has been used by them. However, in this study, we used Pet-28a expression vector. Pet vectors have the advantages of carrying the His tag sequences. The His.Tag sequence binds to divalent captions (e.g., Ni2+) immobilized on the His Bind metal chelation resin [21] which provide a favor conditions for purification. We decided to use an expression plasmid with a short His fusion tag to the recombinant protein to prevent possible nonspecific reaction of rGRA7 and proteins of serum in ELISA experiments and increase the purity of recombinant protein. This recombinant protein was used in serodiagnosis of animal toxoplasmosis (Izatnagar isolate).
Velmurugan et al. [16] expressed SAG1 and GRA7 in pET-32(b) and pET-32(c) as His-tag-thirodoxin fusion proteins, in an insoluble form and transformed into BL21. They used from these recombinant proteins in serodiagnosis of goat toxoplasmosis (Izatnagar isolate). In their study, sensitivity and specificity for rGRA7 was 80.0% and 88.4%, respectively. This study confirmed our results that carried out on human sera. In our study, rGRA7 was expressed in large quantities to earlier studies [12]. The high yield obtained was due to controlled expression of the cloned gene as well as the use of TOP10F E. coli that provided high transformation efficiency and is ideal for high-efficiency cloning and plasmid propagation.
In our results, ELISA using GRA7 antigen showed a considerably higher sensitivity to sera from humans with IgM positive sera than to those from patients with IgG positive sera. Therefore, it seems likely that at least some epitopes presented by GRA7 play an important role in the antibody response of the human host during acute toxoplasmosis. GRA7 is secreted from bradyzoites, constant rupture of the cyst cells, explains why IgG antibody response against GRA7 is high. Consequently, a strong antibody response to this antigen would be expected in sera from chronic patients. In conclusion, ELISA using rGRA7 appears to be a useful method for the diagnosis of acute toxoplasmosis. Identifying different antigens from other strains of T. gondii could be useful for developing recombinant antigen technology in the future.
ACKNOWLEDGMENTS
This study was financially supported by Tehran University of Medical Sciences, Tehran, Iran. We declare that we have no conflict of interest.
