In Vitro Maintenance of Clonorchis sinensis Adult Worms
Article information
Abstract
Clonorchis sinensis is a biological carcinogen inducing human cholangiocarcinoma, and clonorchiasis is one of the important endemic infectious diseases in East Asia. The present study investigated survival longevity of C. sinensis adult worms in various in vitro conditions to find the best way of keeping the worms longer. The worms were maintained in 0.85% NaCl, 1×PBS, 1×Locke's solution, RPMI-1640, DMEM, and IMDM media, and in 1×Locke's solution with different supplements. All of the worms died within 3 and 7 days in 0.85% NaCl and 1×PBS, respectively, but survived up to 57 days in 1×Locke's solution. The worms lived for 106 days in DMEM, and 114 days in both RPMI-1640 and IMDM media. The survival rate in RPMI-1640 medium was the highest (50%) compared to that in DMEM (20±10%) and in IMDM (33.3±25.2%) after 3 months. The 1×Locke's solution with 0.005% bovine bile supplement showed increased duration of maximum survival from 42 days to 70 days. Higher concentration of bile supplements than 0.005% or addition of glucose were disadvantageous for the worm survival. The worms died rapidly in solutions containing L-aspartic acid, L-glutamic acid, and adenine compared to L-arginine, L-serine, and L-tryptophan. In conclusion, the 1×Locke's solution best supports the worms alive among inorganic solutions for 57 days, and the RPMI-1640 medium maintains living C. sinensis adults better and longer up to 114 days in vitro than other media.
INTRODUCTION
Clonorchis sinensis is a carcinogenic liver fluke inducing cholangiocarcinoma in humans. Clonorchiasis, the infection of C. sinensis, is usually symptomless, and this situation makes it commonly neglected. However, it can be fatal quite lately because of its long-term complication of cholangiocarcinoma [1,2]. It is currently estimated that more than 200 million people are at risk of infection, 15-20 million people are infected, and 1.5-2.0 million show symptoms or complications by clonorchiasis. Clonorchiasis is one of the important food-borne neglected tropical diseases in Asia [3].
Studies on several aspects of C. sinensis have investigated this fluke and its infection, but it is still unable to keep its life cycle in the laboratory [4-8]. The in vitro maintenance of the whole life cycle of this fluke may help its study greatly by supplying the materials within the laboratory. In vitro maintenance of C. sinensis worms has used 0.85% normal saline (NaCl) or 1×PBS, mostly to collect excretory-secretory products (ESP) [9-12]. Normal saline and PBS lack essential nutrients and the worms in those solutions may pass all reserved ESP and eggs but not produce new ESP. Therefore, keeping the worms alive in those solutions is possible only for a few days. Li et al. [13] kept newly existed juvenile worms of C. sinensis in saline solution and 1×Locke's solution. The worms in saline died within a few hours but those in 1×Locke's solution lived much longer. The 1×Locke's solution includes CaCl2 in addition to some inorganic components of 1×PBS. The addition of CaCl2 must have boosted diverse cellular function, including cytoskeleton maintenance, cellular signaling, etc. [14,15]. Though the 1×Locke's solution has no nutrients, it looks promising for a long maintenance of C. sinensis adult worms among inorganic solutions. Of course, nutrient supplement may extend the maintaining duration, but no studies have yet tried on its in vitro maintenance.
In this context, the present study investigated long-term maintenance of C. sinensis adult worms in different inorganic solutions and nutrient media to observe the best condition and longevity of in vitro maintenance.
MATERIALS AND METHODS
Collection of adult worms
Metacercariae of C. sinensis were collected from naturally infected fish Pseudorasbora parva following the procedure described by Li et al. [16]. The metacercariae were preserved in 1×PBS with antibiotics (penicillin 100 µg/ml and streptomycin 100 U/ml) at 4℃, and used for the animal infection. Rabbits and rats were infected with 500 and 50 metacercariae, respectively, by intragastric intubation with specific gavage needles. After 8 weeks of infection, the rabbits and rats were sacrificed, and the adult worms were collected. A total of 890 healthy adult worms were selected and washed in sterile 1×PBS for 3 times before placing into culture wells. Then, antibiotics were added to the respective culture solutions or media. The collected healthy worms from rabbits were used for long-term survival in different solutions and media, and those from rats were used for maintenance in solutions with several supplements in 1×Locke's solution. The animal experiment was reviewed and approved by the institutional animal care and use committee of Seoul National University (2010).
Reagents
All of the reagents used in this experiment were purchased from Sigma-Aldrich Co. (St. Louis, Missouri, USA), such as bovine bile, bile salts, conjugated bile salt, amino acids, glucose, etc. Chemicals that were not water soluble were dissolved in dimethyl sulfoxide or alcohol [13].
Experimental design
The collected adult worms were acclimatized through incubation at 25%, 50%, 75%, and 100% concentrations of various test solutions or media for about 1 hr. Worms then incubated at 37℃ with 5% CO2 in 6-well culture plates. A total of 10 worms were kept in a single well with 3 ml of test solution or medium. All of the experiments were repeated in triplicates. Numbers of live worms were counted after 3, 6, and 12 hr, 1, 3, 5, and 7 days, and then every week or every other week by the end of study. Culture media were replaced in every 3 days interval. Gross motility of the worms was used as the criteria for the determination of alive or dead. Non-motile worms were stimulated for its survival using a 26-gauze needle, and those with no response were considered as dead.
Test solutions or media and conditions
Both inorganic solutions (0.85% NaCl, 1×PBS or 1×Locke's) and commercial nutrient media, including Roswell Park Memorial Institute-1640 medium (RPMI-1640), Dulbecco's modified Eagle's Medium (DMEM), and Iscove's Modified Dulbecco's Medium (IMDM) were used for the in vitro maintenance of C. sinensis adult worms with antibiotics. The 1×Locke's solution of the following composition was used in the present study for 1 L of solution: NaCl 8.9 g, KCl 0.42 g, NaHCO3 0.2 g, and CaCl2 0.24 g (wt/vol). Bile, bile salts, glucose, and amino acids were prepared in 1×Locke's solution of different concentrations. Bovine bile was added in concentrations of 0.005%, 0.05%, 0.1%, and 1%. Bile salts were given in 100 µM concentration, which included non-conjugated cholic acid (CA), deoxycholic acid (DCA), lithocholic acid (LCA), and conjugated sodium taurochenodeoxycholate (NTCDC). For a higher concentration of bile, the worms were preconditioned in a lower concentration for 3 hr. LCA was given in additional 1, 5, 20, and 50 µM concentrations as it was found toxic for newly excysted juveniles of C. sinensis [13]. Glucose media were prepared in the following concentrations: 0.1%, 0.5%, and 1%. Amino acids (L-arginine, L-serine, L-tryptophan, L-aspartic acid, and L-glutamic acid) and adenine were added in a concentration of 0.1%.
Statistical analysis
The Kaplan-Meier analysis was performed to prepare survival curves using SPSS 19 software. For the determination of significant difference, Student's t-test was performed. All of the tests were 2-tailed and used 5% level of significance.
RESULTS
Survival of C. sinensis adult worms in different solutions and media
Survival of C. sinensis adult worms was observed in 6 different in vitro solutions and media, including 3 inorganic solutions (0.85% NaCl, 1×PBS, and 1×Locke's) and 3 commercially available nutrient media (RPMI-1640, DMEM and IMDM). Among inorganic solutions, all of the worms died within 3 days in 0.85% NaCl and 7 days in 1×PBS, but a part of them survived up to 57 days in 1×Locke's solution. In 1×Locke's solution, the worms survived much longer than in other inorganic solutions. Among nutrient media, the worms survived up to 106 days in DMEM and 114 days in RPMI-1640 and IMDM media (Fig. 1). The survival rate decreased significantly in 0.85% NaCl at day 1 (50.0±0.0%; P<0.001) and 1×PBS at day 5 (30.0±20.0%; P<0.001) compared to other solution or media used in this study (1×Locke's solution, 86.7±11.5%, P=0.013; RPMI-1640, 90.0±10.0%, P=0.009; DMEM, 93.3±11.5%, P=0.008; and IMDM, 93.3±11.5%, P=0.008). Up to 21 days, the survival rate was over 80% in 1×Locke's solution, RPMI-1640, DMEM, and IMDM. No significant difference was observed between 1×Locke's solution and other nutrient media (RPMI-1640, DMEM, and IMDM) up to 36 days. RPMI-1640 showed the highest survival rate in almost every time points followed by IMDM, DMEM, and 1× Locke's solution (Fig. 1).
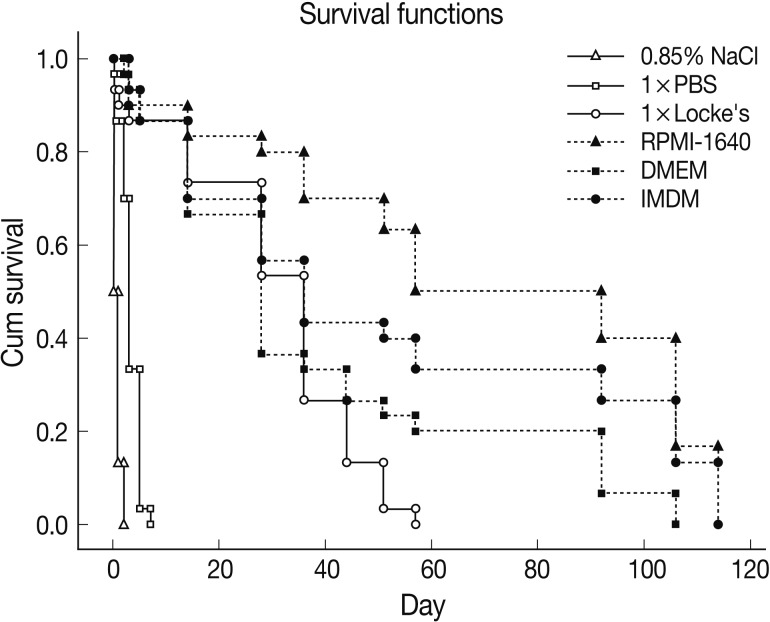
Survival of C. sinensis adult worms in different inorganic solutions and nutrient media. The worms were recovered from experimentally infected rabbits. Worms survived less than 3 days in 0.85% NaCl and up to 7 days in 1×PBS. RPMI-1640 showed the best survival rate among the media studied. Among inorganic solutions 1×Locke's showed the longest survival up to 57 days.
Survival of C. sinensis in bovine bile
Though the worms live in the bile duct and endure in bile, higher concentrations of bovine bile were harmful to them (Fig. 2). All of the worms died within 3 days in 1% bile, and their survival rates significantly decreased to 10% in 0.1% bile solution in a day (P<0.001). The worms survived for 14 days in 0.05% bile solution but survived longer in 0.005% bile solution. The worms survived up to 42 days in 1×Locke's solution and up to 70 days in 1×Locke's solution with 0.005% bile. From day 21 to 42, 0.005% bile supplemented 1×Locke's solution showed significantly higher survival rates (100±0.0% and 86.7±5.7%, respectively) than the control 1×Locke's solution (63.3±23.1% and 13.3±15.3%, respectively) (P<0.001).

Survival of C. sinensis adult worms with different supplements in 1×Locke's solution. All of the worms were recovered from experimentally infected rats. (A) Survival in various concentrations of bovine bile. The worm failed to survive at 1% and 0.1% concentration, however, at 0.005% concentration showed best survival advantages among the concentrations studied. (B) Survival in different concentrations of bile acids. Worm survival rate decreased rapidly in LCA after 7 days. The survival gradually decreased in CA and NTCDC supplemented solutions. (C) Survival in different concentrations of LCA. At lowest concentration (1 µM) studied in this experiment, the worms survived up to 28 days. (D) Survival in different concentrations of glucose. Survival rate decreased very quickly in 1% and 0.5% glucose supplemented solutions just after 3 days. The worms were maintained up to 28 days in 0.1% solution.
Survival of C. sinensis in bile salt solutions
Individual bile acid plays different roles for the survival of adult worms. A previous study [13] showed the survival of C. sinensis newly excysted juveniles in different bile salts. Most of the selected bile acids did not show any significant difference up to day 14 except LCA (P<0.001) (Fig. 2). At day 21, DCA and NTCDC showed significantly lower survival rate (43.3±20.8% and 46.7±25.2% respectively; P=0.013 and P=0.024, respectively) compared to the control solution (63.3±23.1%). CA showed no significant effect on worm survival on day 35 (36.7±5.7%; P=0.067). LCA looked toxic to the adult worms when given at similar concentration of other bile acids included in this study. To see the toxic effect of LCA, different concentrations from 1 to 100 µM were prepared in 1×Locke's solution (Fig. 2). Up to 14 days, the worms survived in different concentrations of LCA without any significant difference except LCA at 100 µM concentrations (10.0±10.0%; P<0.001). However, the survival rate dropped significantly (20.0±17.3% in 1 µM LCA and 0% in 5, 20, and 50 µM LCA) after 21 days in all studied concentrations of LCA compared to 1×Locke's solution only (50.0±10.0%) (Fig. 2).
Survival of C. sinensis in glucose solutions
Glucose supplements in 1×Locke's solution failed to provide any survival advantages to the C. sinensis adult worms (Fig. 2). Their survival rate fell very sharply in 1% and 0.5% glucose solutions after 3 days (16.7±15.3% and 10.0±10.0% respectively; P<0.001 for both), and all of the worms died by 14 days. In 0.1% glucose solution, the survival rate was reduced significantly at day 7 (56.7±32.1%; P<0.001), however, the worms were alive up to 28 days (16.7±11.5%; P=0.067).
Survival of C. sinensis in amino acids and adenine
The present study tested 5 amino acids and adenine for the maintenance of adults. L-aspartic acid, L-glutamic acid, and adenine were found highly toxic to the worms at 0.1% concentration and all of them died less than 1 day (P<0.001). Up to 14 days, no significant difference was observed among media of L-arginine, L-serine, and L-tryptophan supplement (data not presented). All of the worms were dead within 35 days.
DISCUSSION
The present study examined in vitro maintenance of C. sinensis adult worms in inorganic solutions, commercial nutrient media, and 1×Locke's solution with several supplements. The 1×Locke's solution demonstrated the best survival up to 57 days among 3 inorganic solutions, and the RPMI-1640 medium was the best among nutrient media to maintain them alive up to 114 days. One previous study observed survival of C. sinensis newly excysted juveniles in different media [13]. The C. sinensis juveniles survived for 72 hr in 1×Locke's solution, RPMI-1640, DMEM, NCTC-109 and Eagle's media without any significant difference. In 0.85% NaCl and 1×PBS solutions, the survival rate began to drop from 8 hr after incubation, and all of the worms died by 64 hr. Those findings suggest that adult worms may survive much longer than juveniles in cultivation media.
Several studies have dealt survival of flukes in media, such as Maritrema novaezealandensis [17], Echinostoma caproni [18], Paramphistomum spp. [19], and Cymatocarpus solearis [20]. M. novaezealandensis juvenile worms were cultured up to 5 days in NCTC-109 medium with or without serum supplements but the media showed no significant differences [17]. Tonic effect of 1× and 0.5×Locke's solutions was observed by Fried et al. [18] on E. caproni juvenile, and 85% and 55% survival was found respectively after 8 hr. Paramphistomum spp. adults were maintained in Hedon-Fleig, Rohrbacher, and RPMI-1640 media and lasted for 11 days in Rohrbacher medium [19]. Furthermore, ovoculture technique were applied to culture C. solearis from metacercarial stage to adult and the authors recovered only 6 adults with eggs in the uterus after 24 days from about 100 metacercariae [20]. Compared with those previous data, the present study confirmed that Locke's solution and RPMI-1640 media could maintain C. sinensis adult worms much longer than other inorganic solutions or nutrient media. However, it is difficult to compare the results by helminth species because the worm condition and cultivation milieu were quite different.
The 1×Locke's solution provided the best survival of adult worms among the inorganic solutions. The survival rate in 1×Locke's solution was similar with that in nutrient media for more than 1 month. The 0.85% NaCl contained only one salt, whereas in 1×PBS, KCl and phosphates (Na2HPO4, KH2PO4) were present in addition to NaCl. The 1×Locke's solution included carbonate salt (NaHCO3) and another additional inorganic salt, CaCl2 in addition to NaCl and KCl. The worms survived for 57 days in 1×Locke's solution while all of them died within 3 days in 0.85% saline and 7 days in 1×PBS. Calcium is known be important to keep the eukaryotic cells alive [15]. The worms might have survived more than 50 days in inorganic solutions without any nutrient when calcium was supplemented. The finding suggested that carbonate and calcium chloride played an important role for in vitro survival of C. sinensis adult worms.
Among commercial nutrient media, the worms survived up to 106 days in DMEM and 114 days in RPMI-1640 and IMDM. Though both RPMI-1640 and IMDM showed the same longevity, the survival rates were significantly higher in RPMI-1640 than those in IMDM (Fig. 1). RPMI-1640 contained Na2HPO4.7H2O (5.64 mM) whereas DMEM and IMDM contained NaH2PO4.H2O (1.04 mM) instead. RPMI-1640 had very low concentration of glucose (11.1 mM) as well as amino acid, L-hydroxyproline (152.3 µM) and p-aminobenzoic acid (7.29 µM) in comparison with DMEM and IMDM. On the other hand, IMDM showed significantly higher survival than DMEM after 1 month, indicating that the compositional difference between IMDM and DMEM played a role on the survival of C. sinensis adult worms. Besides the common constituents, DMEM contained Fe(NO3)3.9H2O, Ca(NO3)2. 4H2O and IMDM contained CaCl2, KNO3, Na2SeO3.5H2O, biotin and several amino acids in addition. As the worms survived well in IMDM, either beneficial effect of additional components of IMDM or inhibitory effect of additional components of DMEM could be noted. All of the commercial nutrient media had a long list of inorganic and organic components, and it was difficult to identify their effects individually. In that respect, survival of C. sinensis adults for 57 days in inorganic 1×Locke's solution was excellent (Fig. 1), which may have been supported by utilization of calcium [14]. Nonetheless, nutrient media were definitely advantageous for the long-term maintenance of the worms in vitro. We can choose the nutrient media or inorganic solution for keeping the worms long according to the purpose.
Bile has multiple physiological roles in the body. As the worms thrive in bile, bile supplementation was tested in 1×Locke's solution. The solution with 0.005% bovine bile increased the worm survival significantly while the less worms survived in solutions of higher concentration of bile. Optimal absorption or utilization of calcium present in 1×Locke's solution may have been facilitated by bile, which might be the underlying mechanism of increased survival of C. sinensis adult worms by 28 days [15]. Concentrations of bile higher than 0.005% were recognized as disadvantageous to the worms.
Bile salts showed a different effect on the survival as observed previously [13,18]. CA showed little advantages whereas LCA was toxic. Lowest concentration of LCA (1 µM) demonstrated a significant reduction of survival. The similar toxic impact of LCA was observed in C. sinensis newly excysted juveniles [13]. LCA is derived enzymatically from chenodeoxycholic acid and usually present in both human and bovine bile as a minor constituent [21-23]. Thus the worms were unable to tolerate LCA when it was present in higher concentrations (5-10 µM). The toxic effect of LCA to the worms may be through contraction stimulation of the muscular system [13]. The present study found glucose as disadvantageous in several concentrations ranging from 0.1% to 1%. There have been several conflicting data of survival rates observed when glucose was supplemented in the media. Some studies showed the survival advantages of glucose whereas others found it as disadvantageous. The effects of exogenous glucose in artificial spring water (ASW) were studied by Fried et al. [24]. They found that the mean percent survival of Schistosoma mansoni cercariae maintained in 1% glucose in ASW was significantly greater than that of ASW only. Fried et al. [18] and Ponder and Fried [25] found extended survival for excysted metacercariae and cercariae of E. caproni in glucose media. On the contrary, several studies showed the negative effect of glucose on the life span on Caenorhabditis elegans [26-28]. Lee et al. [27] found that adding a small amount of glucose to the medium shortened the life span of C. elegans by inhibiting the activities of life span-extending transcription factors. Moreover, the best nutrient medium in the present investigation, RPMI-1640, contained very small amount of glucose (152.3 µM) compared to DMEM and IMDM (342.7 µM). Glucose was advantageous for the long maintenance only in a very low concentration.
Different studies found different effects of amino acids on the survival. A concentration of 0.1% of L-aspartic acid, L-glutamic acid and adenine reduced survival in C. sinensis newly existed juvenile [13]. The same amino acids in same concentration were toxic also for the adult worms as observed in the present study. Glutamate may kill the worms by neuronal injury [29], however, the mechanism of the damage by L-aspartic acid and adenine is unknown. In the present investigation, no significant change in the survival was observed after 2 weeks in L-arginine, L-serine, and L-tryptophan supplemented media. Most of the amino acids had no significant advantageous effect on the survival of newly excysted juvenile of C. sinensis [13] or E. caproni [18]. Therefore, very low concentrations of amino acids may be an important factor for in vitro survival of worms as observed in nutrient media.
There are a few limitations in the present study. Because of the requirements of large number of C. sinensis healthy adult worms, the present study used limited ranges of concentrations of different supplements, limited number of bile components, and a few selected amino acids. The present study also lacked the information regarding worm morphology and physiology other than the survival. The worms in the present study were in vitro maintained for survival only, not for growth or reproduction. In the present study, any host factor was neglected. The worms used for survival comparison in inorganic solutions and nutrient media (Fig. 1) were recovered from rabbits and those used for supplementation study in 1×Locke's solution (Fig. 2) were from rats. Since the impact on the physiology of C. sinensis by host species is unknown, it is difficult to discuss any difference of their survival by the host. Only the present study used grossly healthy and actively moving worms of 8 weeks age from rabbits or rats. Further studies on in vitro growth or development of C. sinensis adult worms are recommended.
In conclusion, the 1×Locke's solution best supports the worms alive among inorganic solutions for 57 days, and the RPMI-1640 medium maintains living C. sinensis adults better and longer up to 114 days in vitro than other media. The worms survive more when the 1×Locke's solution is supplemented with 0.005% bovine bile.