Trichinella spiralis Infection Suppressed Gut Inflammation with CD4+CD25+Foxp3+ T Cell Recruitment
Article information
Abstract
In order to know the effect of pre-existing Trichinella spiralis infection on experimentally induced intestinal inflammation and immune responses, we induced colitis in T. spiralis-infected mice and observed the severity of colitis and the levels of Th1, Th2, and regulatory cytokines and recruitment of CD4+CD25+Foxp3+ T (regulatory T; Treg) cells. Female C57BL/6 mice were infected with 250 muscle larvae; after 4 weeks, induction of experimental colitis was performed using 3% dextran sulfate sodium (DSS). During the induction period, we observed severity of colitis, including weight loss and status of stool, and evaluated the disease activity index (DAI). A significantly low DAI and degree of weight loss were observed in infected mice, compared with uninfected mice. In addition, colon length in infected mice was not contracted, compared with uninfected mice. We also observed a significant increase in production of pro-inflammatory cytokines, IL-6 and IFN-γ, in spleen lymphocytes treated with DSS; however, such an increase was not observed in infected mice treated with DSS. Of particular interest, production of regulatory cytokines, IL-10 and transforming growth factor (TGF)-β, in spleen lymphocytes showed a significant increase in mice infected with T. spiralis. A similar result was observed in mesenteric lymph nodes (MLN). Subsets of the population of Treg cells in MLN and spleen showed significant increases in mice infected with T. spiralis. In conclusion, T. spiralis infection can inhibit the DSS-induced colitis in mice by enhancing the regulatory cytokine and Treg cells recruitment.
Inflammatory bowel disease (IBD) is a serious, commonly occurring chronic inflammatory condition of the gut [1]. Definite causes of this disease remain unknown; however, according to the current hypothesis, IBD results from an uncontrolled immune response to normal gut flora [2,3]. Both genetic factors and environmental factors contribute to the damaging mucosal immune response [4]. Although a complete explanation of how environmental factors play a role in autoimmune diseases has still not been determined, findings from epidemiologic studies have indicated a link between parasitic infection and its protective role in the development of immune diseases [1,5]. During infection, worms evoke strong immunoregulation, protecting themselves against host responses, for example, by eliciting production of regulatory cytokines, such as IL-10 and transforming growth factor (TGF)-β [6]. According to some previous reports, natural exposure to helminths can afford protection from immunological diseases, and the suppressive effect was dependent on the activity of IL-10 or forkhead box P3 (FoxP3)+ T (Treg) cells [7,8].
T. spiralis is a zoonotic pathogen that infects a wide range of mammalian hosts, including rats, pigs, bears, and humans. During their life cycle, larvae travel via the blood stream and enter skeletal muscles, where each larva invades a single, terminally differentiated muscle cell, synthesizes a collagen capsule, and forms a capillary net around the altered cell [9,10]. As a result of its invasion into muscle cells, T. spiralis can have an influence on the immune system of the host, and T. spiralis infection resulted in prevention of certain immune diseases, including allergic asthma, in experimental animal models [11]. The dextran sulphate sodium (DSS) induced murine models of intestinal inflammation are well-established [12-14]. Especially, the DSS induced colitis model is an acute, chronic, or relapsing model, this model can be produced easily, and dysplasia resembles the clinical course of human ulcerative colitis [14]. In addition, significantly increased expression of inflammatory cytokines (TNF-α, IL-1β, IFN-γ, and IL-6) in colon is characterized in this colitis model [15].
Kang et al. [16] recently demonstrated enhanced production of IL-10 and TGF-β and proliferation of Treg cells in mice infected with T. spiralis. In this study, using an experimental animal model of DSS-induced intestinal inflammation, we evaluated the inhibitory effect of T. spiralis infection on inflammation and the role of CD4+CD25+Foxp3+ T cells.
The T. spiralis strain (isolate code ISS623) used in this study was maintained in our laboratory via serial passage in rats. Female C57BL/6 mice at the age of 6 weeks were purchased from Samtako Co. (Gyeonggi-do, Korea) and maintained during the experimental period in a SPF facility at the Institute for Laboratory Animals of Pusan National University. This study included 2 groups (5 mice each); 1 group was infected with T. spiralis, and the other was not infected. For the infection experiment, C57BL/6 mice received oral infection with 250 infectious T. spiralis larvae, and colitis was induced at week 4 post-infection (PI).
Mice in the colitis induction group received administration of 3% (wt/vol) dextran sulfate sodium (DSS, molecular weight, approximately 40,000; MP Biomedicals, LLC, France) in drinking water from day 0 to day 4. Mice were weighed and observed visually for rectal bleeding and diarrhea every day, beginning on day 0. The disease activity index (DAI) was used for assessment of the grade of colitis, according to a previous report [17]. Samples of colon tissue were isolated from mice; washed with PBS, fixed with 4% paraformaldehyde (PFA), embedded in paraffin, sectioned on glass slides, and stained with heamatoxylin and eosin. For histologic evaluation of tissue damage, microscopic evaluation and scoring of areas of inflammatory lesions was performed in accordance with a previous report [17]. For evaluation of cytokine production ability of lymphocytes, spleen and mesenteric lymph nodes (MLN) were isolated from mice. Methods described in a previous study were used for isolation of lymphocytes [17].
ELISA tests for measurement of IL-6, IL-10, TGF-β, and IFN-γ in culture supernatants of lymphocytes were performed using an ELISA kit (eBioscience, San Diego, California, USA). The assay was performed according to the manufacturer's recommended protocols. For evaluation of recruitment of Treg cells, lymphocytes isolated from spleen and MLN were subjected to CD4, CD25, and Foxp3 staining using FITC-labeled anti-mouse CD4, APC-labeled CD25, and Pacific Blue-labeled Foxp3 antibodies (eBioscience). A FACSCanto™II (BD) with FACS Diva™ 6.0 software was used in performance and analysis of flow cytometry. Qiazol (Qiagen, Hilden, Germany) was used for isolation of total RNA, and the Moloney murine leukemia virus reverse transcriptase kit (Promega, Madison, Wisconsin, USA) was used in accordance with the manufacturer's instructions for performance of reverse RNA transcription. Quantitative expression of cytokine gene mRNAs in colon tissue was evaluated via real-time PCR, and was conducted in accordance with the manufacturer's recommended protocols. All experiments were conducted in triplicate. Calculation of means±SDs was performed and the Student's t-test or ANOVA was used for determination of significant differences. PASW 18.0 was used for statistical analysis.
Most DSS-treated mice suffered temporary severe diarrhea and loss of body weight, and the loss of body weight was recovered within 3 or 4 days after occurrence of symptoms. Body weight of mice in the T. spiralis-infected group showed a more rapid recovery, compared with uninfected mice (Fig. 1A). As a result of persistent diarrhea, the shrunken colon length was observed. After sacrifice of animals, colon lengths of uninfected mice were found to be shorter than those of infected mice (Fig. 1B). In addition, we evaluated the severity of colitis based on the percentage of weight loss, changes of stool states, including consistency, and presence of blood in stool of mice [17]. In most of the mice, DAI value showed an increase from day 3 and decreased after day 8, however, a significantly lower DAI value was observed in mice infected with T. spiralis, compared with those of uninfected mice on days 7 and 8 (Fig. 1C). In colons of mice not treated with DSS, intact structures of epithelium and submucosal layer were observed in T. spiralis-infected and uninfected mice (Fig. 1Da & Db). However, submucosal layers of the colon showed various inflammatory cell infiltration and ulcerative mucosa, as well as destruction of villi in the epithelium, in mice treated with DSS (Fig. 1Dc). However, greater amelioration of the mucosa was observed in T. spiralis-infected mice treated with DSS; in particular, immune cell infiltration, ulceration, and inflammation showed a significant decrease, compared with uninfected mice treated with DSS (Fig. 1Dd). Scores for tissue histopathology index were significantly lower in mice infected with T. spiralis, compared with uninfected mice (Fig. 1E).

T. spiralis infection could inhibit DSS-induced intestinal inflammation. Four weeks after T. spiralis infection or uninfection, mice were treated with 4% DSS for a period of 4 days. Weight change during experimental period was expressed as percentage change from day 0 (A). Colon lengths after 4 days of DSS treatment was measured (B). Scoring of weight loss, stool consistency, and fecal blood was performed in order to provide DAI for each group (C). Histopathological changes in large intestines from colon are shown in (D). Uninfected mice, a and c; infected mice, b and d; DSS untreated mice, a and b; DSS treated mice, c and d. Inflammatory score for the colon was calculated (E). *P<0.05, **P<0.01, ***P<0.001, n=5 mice/group, 3 independent experiments.
For evaluation of cytokine production in lymphocytes of spleen MLN and, ELISA was performed for detection of pre-inflammatory cytokine (IL-6), Th1 cytokines (TNF-α and IFN-γ) and regulatory cytokines (IL-10 and TGF-β). Of particular interest was that the levels of IL-6 and IFN-γ showed a significant increase in spleen and MLN treated with DSS. These increases were reduced by T. spiralis infection (Fig. 2). In addition, levels of IL-10 and TGF-β showed a greater increase in mice infected with T. spiralis, compared with those of uninfected mice (Fig. 2). IL-10 and TGF-β showed a close association with activation and differentiation of Treg cells.
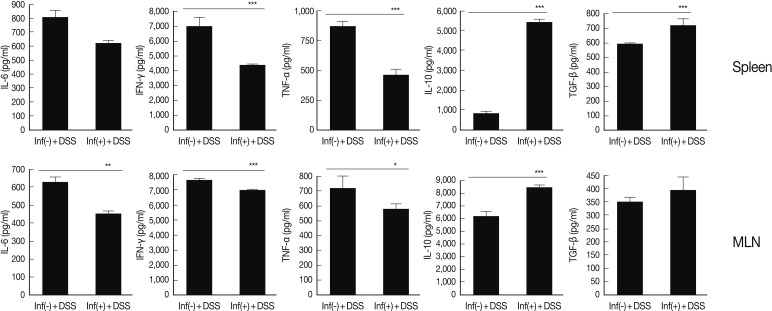
Comparison of Th1 and regulatory cytokines level between spleen and MLN of T. spiralis infected and uninfected mice. After isolation of lymphocytes from spleen and MLN, the cells were stimulated with anti-CD3 antibody. After 72 hr, cytokines levels in the supernatant were measured. *P<0.05, **P<0.01, ***P<0.001, n=5 mice/group, 3 independent experiments.
In this study, the population of Treg cells was observed at 5.2% (±0.42) and 7.2% (±0.17) in the spleen of uninfected and T. spiralis-infected mice treated with DSS, respectively. In MLN of uninfected mice and T. spiralis-infected mice treated with DSS, a Treg cell population of 3.7% (±0.70) and 6.66% (±0.89) was observed (Fig. 3A). In order to determine inflammation-related gene expression level of the large intestine, measurements of IFN-γ and TNF-α were performed. Levels of expression showed a significant increase by DSS treatment; however, those of mice infected with T. spiralis showed a significant decrease. Gene expression levels of IL-10, TGF-β, and Foxp3 in intestinal tissues showed an increase in mice infected with T. spiralis, compared with uninfected mice (Fig. 3B). These results suggested greater recruitment of Treg cells, and/or IL-10, and TGF-β secreted cells to intestinal tissue in mice infected with T. spiralis.

Treg cell recruitment in spleen and MLN by T. spiralis infection and Th1 & Treg cell related gene expression levels in large intestinal tissue. The populations of Treg cell were calculated in MLN and spleen of T. spiralis-infected and uninfected mice after inflammation induction (A). *P<0.05, **P<0.01, ***P<0.001, n=5 mice/group, 3 independent experiments. Total RNAs were isolated from T. spiralis-infected and uninfected mice colon to real time-PCR analysis for evaluation of the expression of Th1 related genes (IFN-γ and TNF-α) and Treg cell related genes (IL-10, TGF-β, and Foxp3) (B).
In this study, we induced intestinal inflammation by treatment of T. spiralis-infected and uninfected mice with DSS. The majority of the results suggested more rapid and/or effective amelioration of induced inflammation in the parasite infected group. This DSS inflammation model is known to be closely related to Th1 cytokines, such as TNF-α, IFN-γ, and IL-1β, which were found to be important inflammatory mediators in human IBD and the DSS-induced colitis mouse model [18-20]. In particular, TNF-α is a key cytokine in the pathogenesis of IBD. Therefore, the most useful therapeutic agents against IBD were antibodies against TNF-α cytokines, including infliximab, adalimumab, and certolizumab [21,22]. However, this medication can cause serious side effects, which usually occur, therefore, therapy should be monitored carefully [23,24]. New therapeutic methods should be continually investigated. According to recent reports, severity of subsequent colitis is attenuated by helminthic infections, including Schistosoma mansoni and Trichuris suis; therefore, a therapeutic role for helminthic infections in IBD has been suggested [25,26].
We demonstrated that T. spiralis infection exerts anti-inflammatory effects with regard to the inhibition of epithelial cells and crypt cell destruction and inflammatory responses in the DSS-induced colitis (Fig. 1). In addition, we observed that levels of production of most Th1 cytokines in lymphocytes were significantly lower in the group of mice with T. spiralis infection; by contrast, levels of regulatory cytokines (IL-10 and TGF-β) showed an increase in infected mice (Fig. 2). IL-10, which is referred to as the cytokine synthesis inhibitory factor, is an anti-inflammatory cytokine that is capable of inhibiting the pro-inflammatory cytokine synthesis. IL-10 is generated primarily by Treg cells, and has been shown to induce Treg cell differentiation [27,28]. TGF-β has also been implicated in conversion of naive CD4+CD25- T cells into CD4+CD25+ T cells via the activity of Foxp3. TGF-β also promotes the in vivo expansion and suppressive functions of CD4+CD25+ Treg cells [29,30]. In addition, in the large intestine, we observed elevated levels of expression of IL-10, TGF-β, and Foxp3 in mice infected with T. spiralis (Fig. 3). These results suggested elicitation of immune regulation (ameliorate inflammation) effects by Treg cells or IL-10 secreted cells in local organs as well as in the entire body system.
In conclusion, T. spiralis infection may have therapeutic potential for use in the treatment of Th1-driven intestinal inflammation. Further studies on the mechanisms and pathways of the protective properties of T. spiralis infection are needed.
ACKNOWLEDGMENT
This work was supported by Mid-Career Researcher Program through NRF grant funded by the Ministry of Education, Science and Technology (MEST) (No. 2010-0027791).