Protective Role of Purified Cysteine Proteinases against Fasciola gigantica Infection in Experimental Animals
Article information
Abstract
Fascioliasis is one of the public health problems in the world. Cysteine proteinases (CP) released by Fasciola gigantica play a key role in parasite feeding, migration through host tissues, and in immune evasion. There has been some evidence from several parasite systems that proteinases might have potential as protective antigens against parasitic infections. Cysteine proteinases were purified and tested in vaccine trials of sheep infected with the liver fluke. Multiple doses (2 mg of CP in Freund's adjuvant followed by 3 booster doses 1 mg each at 4 week intervals) were injected intramuscularly into sheep 1 week prior to infect orally with 300 F. gigantica metacercariae. All the sheep were humanely slaughtered 12 weeks after the first immunization. Changes in the worm burden, ova count, and humoral and cellular responses were evaluated. Significant reduction was observed in the worm burden (56.9%), bile egg count (70.7%), and fecel egg count (75.2%). Immunization with CP was also found to be associated with increases of total IgG, IgG1, and IgG2 (P<0.05). Data showed that the serum cytokine levels of pro-inflammatory cytokines, IL-12, IFN-γ, and TNF-α, revealed significant decreases (P<0.05). However, the anti-inflammatory cytokine levels, IL-10, TGF-β, and IL-6, showed significant increases (P<0.05). In conclusion, it has been found that CP released by F. gigantica are highly important candidates for a vaccine antigen because of their role in the fluke biology and host-parasite relationships.
INTRODUCTION
Fascioliasis is an important human disease caused by Fasciola hepatica and Fasciola gigantica. They parasitize the liver of domestic, wild animals, and humans [1]. In Egypt, the emerging situation of both human and livestock fascioliasis has increased significantly due to both F. gigantica and F. hepatica [2-4]. Worldwide, more than 90 million people are at risk of fascioliasis and between 2.4 and 17 million individuals are infected with Fasciola [5].
A significant data suggests that a number of molecules, including cathepsins L, glutathione S-transferase (GST), leucine aminopeptidase (LAP), and fatty acid binding proteins (FABP) have the potency of inducing a protective response against Fasciola in laboratory animals and large animal models [6,7]. The enzymes belonging to the cysteine proteinase (CP) family have been studied most intensely and have given the most promising results when used as vaccine antigens [6]. These enzymes are involved in feeding, migration, and immune evasion by Fasciola [8-11].
Chronicity and the T-helper 2 (Th2) immune responses are features of helminth infections in humans. The liver fluke promotes its own survival through several strategies to down-regulate the immune response of the host during the early phase of infection. The liver fluke secretes molecules, known as excretory-secretory (ES) products that modulate or suppress host immune responses [12,13]. During early chronic infections, there is a predominance of a Th2 response, which decreases in advanced chronic infections characterized by a persistent immune suppression [14].
CD4+ T cells can be separated into 2 major subsets, Th1 and Th2, on the basis of their cytokine secretion patterns and function. Th1 cells produce many cytokines, including IFN-γ and TNF-α, and promote the activation of macrophages which lead to the production of opsonizing antibodies. Also, Th1 cells promote mediation of a delayed-type hypersensitivity reaction and inflammatory responses. Th2 cells produce many other cytokines, including IL-4, IL-6, and IL-10, and promote immediate-type hypersensitivity reactions, involving IgE, eosinophils, and mast cells [13]. Generally, helminth infections are manifested by suppression of Th1 function and induction of T cells, which express cytokines characteristic of the Th2 subset [15].
Vaccination studies with purified native or recombinant Fasciola antigens suggest that this approach, which diminished morbidity and mortality and reduced transmission, is a realistic goal [16]. However, despite long-standing research, a vaccine against this parasite has not yet been developed to the point of commercialization [17]. This can be largely attributed to a fact that immune responses to vaccines are influenced by the route of immunization (injection or oral), form of antigen, and presence of adjuvant in the vaccine [18,19].
The present study was designed to study the effects of CP as a protective vaccine on the humoral and cellular immune responses in F. gigantica-infected sheep as an attempt to use CP as a vaccine against Fasciola infection.
MATERIALS AND METHODS
Animals
Thirty-two young sheep, 6-month-old, were used in this study. They were proved to be free from any parasitic infections by examining them by both parasitological and ELISA tests [20]. All procedures related to animal experimentation met the International Guiding Principles for Biomedical Research Involving Animals as issued by the International Organizations of Medical Sciences.
Parasites and infection
Metacercariae of F. gigantica were purchased from the Schistosome Biology Supply Center (SBSC) of Theodor Bilharz Research Institute (TBRI), Giza, Egypt. Sheep were infected with 300 F. gigantica metacercariae, via oral route using a dosing gun placed inside gelatin capsules (Torpa Inc., Fairfield, New Jersey, USA) [21].
Preparation of ES products
Adult F. gigantica worms were collected from the biliary tracts and gallbladders of condemned bovine livers from a local slaughter-house. The live intact worms were washed 6 times with cold 0.01 M PBS (pH 7.4) containing 125 mM NaCl for 1 hr to eliminate any traces of bile, blood, and contaminated microorganisms [21]. They were then incubated for 16 hr at 37℃ in RPMI 1640 medium (pH 7.4). Following incubation, the medium was removed and was centrifuged at 15,000 g for 30 min. The supernatant containing ESPs was collected, and the protein content was measured (Bio-Rad, Richmond, California, USA). It was then stored at -20℃ [22].
Purification of F. gigantica cysteine proteinase (CP)
ESPs were concentrated using an Amicon 8400 ultrafiltration unit with membrane (3 kDa cut-off). The sample was applied to DEAE-sephadex A50 column (ion exchange column chromatography) followed by a Sephacryl S-200 HR column (gel filtration chromatography) equilibrated in 0.1 M Tris-HCl, pH 7 [23].
Experimental design
Sheep were divided into 3 groups (8 sheep/group). The first group was a normal control group. The second group was the infected control group; sheep were infected with F. gigantica metacercariae as mentioned before. The third group was the immunized-infected group, where the sheep were immunized intramuscularly 4 times at 2 week interval, with 2 mg of purified CP diluted in 250 µl PBS and 250 µl of complete Freund adjuvant (CFA) in the priming dose and 1 mg of CP diluted in 250 µl PBS and 250 µl of incomplete Freund adjuvant (IFA) in the subsequent booster doses. Two weeks after the last booster dose of immunization, sheep were infected with F. gigantica metacercariae as mentioned previously. Twelve weeks after infection, all the sheep were humanely slaughtered for subsequent assessment of both parasitological and immunological parameters. Whole blood was collected from each sheep and centrifuged at 2,000 rpm at 4℃ for 10 min, and the obtained serum samples were stored at -80℃ until analysis.
Fluke counts
Worms were recovered from the gall bladder and livers from the sheep. The gall bladder was removed, the major bile duct was opened with blunt-blunt scissors and any visible flukes were removed with blunt forceps. Liver of each sheep was turned upside down in a tray and soaked with hot tap water for 1 hr. Finally, the liver was cut into 1-2 cm slices and placed in a 13 liter bucket filled with hot tap water. After allowing standing and stirring, the supernatant was poured through 200 mm sieves to retrieve the flukes. This procedure was repeated twice. Flukes from all 3 washes were collected and counted using a back-lighted magnifier. The maturity of the flukes (juvenile vs adult) was determined based on their size, length, and development of the vitelline glands. The mean total number of flukes/group was calculated [24].
Bile egg count
The collected bile was cleared by repeated sedimentation of the eggs. Egg counts were performed under a light microscope by measuring a definite volume (10 µl×5) of each sample to calculate the total eggs in the given volume of bile as in the standard protocol [25].
Fecal egg count (FEC)
Feces were removed from the rectum of each sheep after slaughter. Four grams of dried feces were diluted in 200 ml of water and resuspended by vortexing. The feces were then filtered through sieves of different pore sizes (300, 150, and 32 mm), and the filtrate was allowed to stand for 30 min after which the sediment was collected in a test tube and centrifuged at 700 g for 3 min. After centrifugation, the supernatant was removed, and the sediment was left to stand at room temperature for 3 min and resuspended in 250 ml polyethylene conical flask (Falcon, USA). The number of F. gigantica eggs was examined microscopically under a 100× magnification [26].
Assessment of anti-Fasciola total IgG, IgG1 and IgG2 by ELISA
Anti-Fasciola total IgG, IgG1, and IgG2 subclasses were measured using the Fasciola antigen (CP) by indirect ELISA, based on the method of Engvall and Perlman [27]. The wells of the ELISA microtitre plate (Costar, Cambridge, Massachusetts, USA) were coated with suspension of 5 µg of Fasciola antigen (CP) (100 µl/well) in binding buffer (0.05 M carbonate buffer, pH 9.6). The sera were diluted 1:200, 1:250, and 1:100 with PBS/T for measurement of total IgG, IgG1 and IgG2 respectively. Anti-goat IgG peroxidase-labeled conjugate, gamma chain-specific (Sigma, St. Louis, Illinois, USA), specific conjugate anti-goat IgG1 and anti-goat IgG2 labeled horseradish peroxidase (HRP) (Sigma) were used at a dilution of 1:1,000, 1:250, and 1:400, respectively. Then, the plates were incubated for 30 min in a water bath at 37℃, washed 5 times with PBS/T and incubated with 100 µl/well of ortho-phenylenediamine (OPD) (Sigma) substrate for 15 min. The reaction was stopped with 50 µl/well of 8N H2SO4, and absorbance was measured as optical density values (OD) at 492 nm using a microplate ELISA reader (Bio-Rad).
Assessment of pro-inflammatory and anti-inflammatory cytokines by sandwich ELISA
Serum levels of IL-12, IFN-γ, TNF-α, IL-10, TGF-β, and IL-6 were measured with an ELISA kit (Quantikine M, R, & D systems, Minneapolis, Minnesota, USA). The detection limit of the assay was consistently 20 pg/ml. The concentration was calculated from the standard curve that was performed in the same assay.
Statistical analysis
Data are expressed as mean±SD. Comparison between the mean values of different parameters in the studied groups was performed using 1-way ANOVA test, with post-hoc using LSD test. The percent reduction in the worm burden was calculated from the equation: (mean number of worms in infected control group - mean number of worms in immunized-infected group)/(mean number of worms in infected control group)×100. The data were considered significant if P-value was≤0.05.
RESULTS
Purification of parasitic antigen
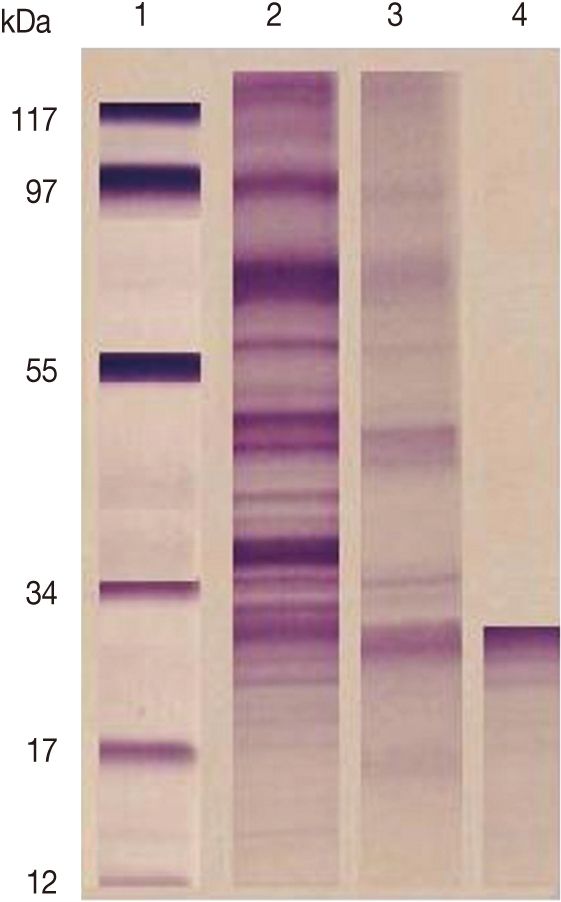
F. gigantica CP was purified from ES products by a combination of gel filtration and ion-exchange chromatography. Analysis by SDS-PAGE (under reducing conditions) revealed that the purified preparations contained single proteins of 27 kDa (Fig. 1).
Fluke weight and number
The mean total number of worms in the challenge group (77.3±18.2) was significantly reduced than their corresponding F. gigantica infected control group sheep (179.2±21.2) (P<0.01) with a percent reduction of 56.9%.
Bile and fecal egg counts
The mean egg count collected from both bile and feces was significantly reduced in the challenge group (P<0.05) when compared with the F. gigantica infected control group, yielding a percent reduction in the bile and fecal egg count of 70.1% and 75.2%, respectively (Table 1).
Serum anti-Fasciola total IgG, IgG1, and IgG2
The level of total IgG, IgG1, and IgG2 was significantly increased in immunized-infected group when compared with their corresponding F. gigantica-infected control group (P<0.05). The OD values of total IgG, IgG1, and IgG2 in the normal control group always remained below the cut-off value of the F. gigantica-infected control group (Table 2).
Pro-inflammatory and anti-inflammatory cytokines
Serum levels of pro-inflammatory cytokines, IL-12, IFN-γ, and TNF-α, were significantly decreased (P<0.05), while the anti-inflammatory cytokine levels, IL-10, TGF-β, and IL-6, were significantly increased (P<0.05) in immunized-infected group when compared to their corresponding F. gigantica-infected control group (Table 3).
DISCUSSION
Due to the importance of peptidases in host-parasite interactions, they are considered to be promising targets for the development of novel chemotherapeutic drugs and vaccines against a number of trematodiases, including schistosomiasis, fascioliasis, paragonimiasis, and opisthorchiasis [28]. The use of Fasciola worm-derived ES proteins, namely CP, for serodiagnosis implies that these molecules elicit strong cellular and humoral immune responses during F. hepatica and F. gigantica natural infection [12,29-31].
Hillyer [16] reported that CP comprise a large family that include cathepsin L and B that have been studied in relation to parasite invasion, feeding, immune evasion, and vaccine potential. The Fasciola cathepsin L protease has been proposed to play a number of functional roles, including promoting tissue penetration, nutrition acquisition, egg production, and immune evasion by cleavage of Fc portion of immunoglobulins [28,32].
In our work, gel electrophoresis showed that the purified CP was 27 kDa. This result is in agreement with Sobhon [33] who reported that gel electrophoresis in 1- dimensional indicated that the most prominent F. gigantica ES proteins were 66, 64, 58, 54, 28, and 26-27 kDa. The former 4 molecules were shown to be derived from the worm tegument, while the latter 28 and 26-27 kDa species appeared to be released from cells lining the gut [16,34]. The 28 and 26-27 kDa molecules were shown to essentially consist of cathepsin CP [35,36].
The reduction of egg fecundity observed in this study may be due to the effect of the CP antigens, which were found to induce reduction in egg fecundity. This result suggested that protective immune responses were of the Th1 type, involving the IgG2 isotype, IFN-γ-activated macrophages, and cytotoxic T cells [16]. Also, Van-Milligien [37] and Harmsen [38] obtained an antigen fraction derived from newly existed F. hepatica juveniles, containing an immune reactive 32 kDa protein. This antigen has 70% sequence homology with cathepsin L1 and L2 which induced almost complete protection to challenge infections with reduction in egg fecundity of over 90% in comparison to naïve control rats.
The immunization of sheep with CP injected intramuscularly induced reduction of the number of adult flukes and bile and fecal egg counts compared to infected controls. The effects of the vaccine on egg production and hatch rate may be mediated by antibodies that inhibit parasite feeding by blocking cathepsin L activity, thereby preventing the acquisition of amino acids for the synthesis of egg proteins. Additionally, cathepsin Ls have been immunolocalized in the reproductive organs of the mature parasite [32,39].
Specific IgG and the 2 subclasses, IgG1 and IgG2, were significantly higher in immunized infected group compared to the infected control group. This result was in agreement with Hoyle [40]. They reported that immune antibody responses to a cathepsin L-based vaccine included high titers of both IgG1 and IgG2 indicating that protection is associated with induction of a Th1 response or mixed Th1/Th2 responses.
Information on the immune status and cytokine profiles of animals infected with F. gigantica is scanty, despite the fact that F. gigantica is an economically important parasite of livestock. A few published studies on cytokine profiles during fascioliasis are on F. hepatica infection in cattle and sheep, which have demonstrated a dominant Th2-type immune response during chronic infections [13,41]. However, some contrasting observations have been reported indicating a Th0 response during early phases of infection wherein IFN-γ, IL-2, and IL-4 were produced [42,43]. Non-polarized Th0 response has also been reported in cattle during a chronic phase of F. hepatica infection [12].
In our study, the serum levels of pro-inflammatory cytokines, IL-12, IFN-γ, and TNF-α, showed significant decreases. However, the anti-inflammatory cytokine levels, IL-10, TGF-β, and IL-6, showed significant increases. The T-cell response during F. gigantica infection was a Th2 biased immune response during early phases of infection [13]. It was also reported that the production of Th2 cytokine via IL-6 in the serum of infected cattle during early phases of infection but absence of IFN-γ during this period of infection [13]. Studies on cytokine expression during F. gigantica infection in cattle are limited.
In line with a helminth's need to suppress inflammatory responses, a reported decrease in proinflammotory cytokines was observed. Since IL-12 is an important polarizing cytokine known to drive Th1 differentiation, the suppression in its level may be due to a Th1 suppression observed during F. gigantica co-infections [44]. At the same time, the reason of the increase of the levels of IL-10, TGF-β, and IL-6 is because that these cytokines are traditionally associated with anti-inflammatory or regulatory responses [45].
In conclusion, it has been found that CP released by F. gigantica are highly important candidates for a vaccine antigen because of their role in the fluke biology and host-parasite relationships. Also, the CP-induced cellular and humoral immune responses were associated with a modest reduction in the worm count, thus suggesting that CP immunization might be a safe and cost-effective strategy for reducing transmission of the infection.
ACKNOWLEDGMENTS
This work was funded by the Yousef Jameel Science and Technology Research Center (STRC), The American University in Cairo, Cairo, Egypt.