Protective and Anti-Pathology Effects of Sm Fructose-1,6-Bisphosphate Aldolase-Based DNA Vaccine against Schistosoma mansoni by Changing Route of Injection
Article information
Abstract
This study aimed to evaluate the efficacy of fructose-1,6-bis phosphate aldolase (SMALDO) DNA vaccination against Schistosoma mansoni infection using different routes of injection. The SMALDO has been cloned into the eukaryotic expression vector pcDNA3.1/V5-His TOPO-TA and was used in injecting Swiss albino mice intramuscularly (IM), subcutaneously (SC), or intraperitoneally (IP) (50 µg/mouse). Mice vaccinated with non-recombinant pcDNA3.1 served as controls. Each group was immunized 4 times at weeks 0, 2, 4, and 6. Two weeks after the last booster dose, all mice groups were infected with 80 S. mansoni cercariae via tail immersion. At week 8 post-infection, animals were sacrificed for assessment of parasitological and histopathological parameters. High anti-SMALDO IgG antibody titers were detected in sera of all vaccinated groups (P<0.01) compared to the control group. Both the IP and SC vaccination routes resulted in a significant reduction in worm burden (46.2% and 28.9%, respectively, P<0.01). This was accompanied by a significant reduction in hepatic and intestinal egg counts (41.7% and 40.2%, respectively, P<0.01) in the IP group only. The number of dead eggs was significantly increased in both IP and IM groups (P<0.01). IP vaccination recorded the highest significant reduction in granuloma number and diameter (54.7% and 29.2%, respectively, P<0.01) and significant increase in dead miracidia (P<0.01). In conclusion, changing the injection route of SMALDO DNA vaccination significantly influenced the efficacy of vaccination. SMALDO DNA vaccination via IP route could be a promising protective and anti-pathology vaccine candidate against S. mansoni infection.
INTRODUCTION
Schistosomiasis is one of the major public health problems in Egypt, especially in Upper Egypt [1]. Emphasis has been placed on chemotherapy as the preferred method of treatment for schistosomiasis. However, control programs based on chemotherapy are complicated by the frequency of reinfection, in addition to development of drug resistant strains. Effective control of schistosomiasis is unlikely in the absence of a vaccine [2].
High levels of protective immunity have been achieved in experimental animals using alive attenuated vaccine [3]. Previously, we have identified the S. mansoni glycolytic enzyme fructose-1, 6-bisphosphate (FBP) aldolase cDNA (1,431 base pair) clone (SMALDO), which has been recognized by protective antibodies taken from rabbits vaccinated with irradiated cercariae [4]. Sera of these rabbits were able to passively transfer high levels of resistance against S. mansoni challenge infection to naïve mice when it was given around the time of the challenge.
Carbohydrate metabolism in schistosomes points to a dominant anaerobic metabolism through glycolysis in vertebrate-dwelling stages of the parasite [5]. FBP aldolase plays a central role in glycolysis by catalyzing the reversible aldol cleavage of FBP into the 2 trioses, dihydroxyacetone-phosphate and glyceraldehyde-3-phosphate [6] and it is important for production of energy required for different schistosome activities and survival [7]. It has 61-70% sequence homology with other FBP aldolases from different species and higher vertebrates (type A aldolase) [4]. It is highly expressed in different life cycle stages [4] and is a potentially suitable target for intervention. This enzyme also induces strong humoral and cell-mediated immune responses and has protective and granuloma-modulating effects in animals vaccinated with the recombinant protein [8,9].
Although the antigens determined following the irradiated cercariae model were characterized using immunologic criteria, and their recombinant polypeptides were tested using different adjuvants, yet the anti-schistosomiasis vaccine has not been developed from this molecule. It may be because many of the vaccination strategies induced mainly humoral responses and insufficient cell-mediated immunity, which is an essential mechanism in protection against schistosomiasis [2,10]. Immunization with DNA could enhance the vaccination efficacy against a wide range of infectious diseases and tumors. Injection of plasmid harboring gene encoding antigen under control of promoter working in an eukaryotic cells, such as cytomegalovirus (CMV) promoter, led to expression of this antigen intracellularly and efficiently induced both humoral and cell-mediated immunity [11].
Several DNA vaccination trials against schistosomiasis have been conducted. Some of them were able to induce both antigen-specific humoral and cell-mediated immune responses, while others managed to get a low degree protection [2,12,13]. In our laboratory, we have developed protective DNA vaccines against S. mansoni using intramuscular injection strategy either for single gene [14] or primed with DNA vaccine and boosted with recombinant protein and multivalent DNA vaccination [15].
The present study aimed to evaluate the efficacy of DNA vaccination using the SMALDO construct administered via different routes of injections and their role in induction of protection against S. mansoni infection as well as accompanying histopathological changes.
MATERIALS AND METHODS
Animals and parasites
Female CD1 Swiss albino mice, weighing 20±2 g, bred and maintained at the Schistosome Biology Supply Center (SBSC) of Theodor Bilharz Research Institute (TBRI), Giza, Egypt, were obtained. Handling and treatment of animals were conducted according to the internationally valid guidelines and ethical conditions. Animals were infected with the Egyptian strain of S. mansoni cercariae using the tail immersion technique [16].
Vectors and bacterial strains
The pGEX-2T-SMALDO vector containing the gene SMALDO in BamHI site and the host Escherichia coli strain DH5 α were used as the source for the cloned gene and in fusion protein production [4]. The eukaryotic expression vector pcDNA3.1/V5-His© TOPO® TA with TOP10 E. coli cells (Invitrogen Co., Carlsbad, California, USA), were used in DNA vaccine construct preparation and propagation.
Primers
Modified primers for cloning of SMALDO gene in pcDNA3.1/V5-His© TOPO were designed as follows: forward: 5'GCCACCATGGCACGCTTCCAACC 3', reverse: 5'GCTATTAATAAGCGTGATTAGC 3'.
Preparation of pcDNA3.1/SMALDO construct
The pGEX-2T plasmid containing the full length SMALDO construct (pGEX-2T/SMALDO) [4] was digested by EcoRV restriction enzyme for linearization of this recombinant plasmid. The open reading frame (ORF) of SMALDO was amplified from this linearized recombinant plasmid using forward and reverse primers, modified for direct cloning of SMALDO into pcDNA3.1/V5-His expression vector, by PCR [17]. The PCR product was purified using an agarose gel DNA extraction kit (Roche Diagnostics GmbH, Manheim, Germany) and was cloned into the pcDNA3.1/V5-His vector using the TOPO cloning technology [18]. The ligated vector was transformed into competent TOP10 E. coli cells. Recombinant transformed colonies, identified by using PCR analysis for the SMALDO gene, were selected for plasmid minipreparation, restriction analysis using BamHI and EcoRV enzymes and DNA sequencing. Two clones with right oriented inframe SMALDO insert and another reverse oriented one, as a vector control, were used for large-scale plasmid preparation, and purified by polyethylene glycol-dependent plasmid preparation method [19], precipitated with 75% ethanol, washed, and dissolved in sterile saline. The DNA construct was sterilized by filtration through 0.2 µm syringe filters and used in DNA vaccination of mice.
Vaccinations with pcDNA3.1/SMALDO construct and infection
Three groups, 10 mice each, were immunized four times on days 0, 14, 28, and 42 with 50 µg of purified right-oriented recombinant plasmid pcDNA3.1/SMALDO via different routes of injections: IM, SC and IP. Mice of the 4th group were injected IM and IP with purified reverse-oriented recombinant clone (50 µg via each route) and served as vector-vaccinated control group [20]. Two weeks after the last immunization, blood was collected from the retro-orbital sinus of each mouse, sera were separated, aliquoted and stored at -20℃ until use. Then, mice were infected with S. mansoni cercariae (80/mouse), eight weeks later all mice groups were sacrificed and different parameters were assessed. This experiment was repeated twice.
Detection of anti-SMALDO IgG antibodies
For evaluation of SMALDO expression and the induction of humoral immunity in animals vaccinated with the pcDNA3.1/SMALDO construct, anti-SMALDO IgG titer was estimated by ELISA in sera of mice of different vaccination groups 2 weeks after the last immunization as follows. Microtitration plates (Thomas Scientific, City, State, Country) were coated with 100 µl/well (5 µg/ml) of recombinant SMALDO (prepared and purified using pGEX expression system according to El-Dabaa et al. [4] in 0.1 M carbonate buffer, pH 9.6, and left overnight at 4℃). Plates were blocked by the addition of 200 µl/well of 2.5% bovine serum albumin (BSA) in 0.02 M PBS with 0.05% Tween 20 (PBS/T) (pH 7.2), for 2 hr at 37℃. Diluted sera (1:500 in PBS containing 2% BSA) were added (100 µl/well) and incubated for 2 hr at 37℃. Plates were washed with PBS/T. Anti-mouse IgG alkaline phosphatase conjugate (Sigma-Aldrich, St. Louis, Illinois, USA) was diluted in PBS/T containing 2% BSA to reach a final concentration of 5 µg/ml and dispensed at 100 µl/well. Plates were incubated for 1 hr at room temperature, and then thoroughly washed with PBS/T. The reaction was visualized by addition of 200 µl/well of P-nitrophenyl-phosphate substrate (Sigma-Aldrich) for 15-30 min in the dark at room temperature. Then, the absorbance was measured at dual wave length (405/540 nm) using the ELISA reader (Microplate Reader, Bio-Rad, Richmond, California, USA) [21].
Assessment of parasitological criteria
Worm burden (count); Hepatic and porto-mesenteric vessels were perfused and worms were recovered and counted [22].
Tissue egg load; The number of eggs per gram of hepatic or intestinal tissue was counted after digestion overnight in 5% KOH [23].
Percentage egg developmental stages "oogram pattern"; The oogram pattern was examined in the small intestine in 3 samples per animal and the mean of each stage/animal was obtained [24]. Three stages of eggs were identified; immature egg (the embryo occupies the entire egg shell), mature egg (a fully-developed miracidium occupies the whole shell), and dead egg (the egg appeares as semitransparent granular and darkened with retracted embryo) [24].
Histopathological examinations
After sacrifice of mice, approximately half of the liver was removed and the specimens were immediately fixed in 10% formalin and embedded in paraffin. Histological sections were processed and stained with hematoxylin-eosin and Masson's trichrome. Sections were examined for the presence of hepatic granulomas and associated histological changes. The diameter (µm) and cell composition of the granulomas surrounding single egg were measured. Only lobular (parenchymal) granuloma around an egg containing a miracidum was measured. Egg viability was assessed microscopically in the same liver sections [25].
Statistical analysis
Results were expressed as means±SD or number (%). Comparison between the mean values of different parameters was performed using 1-way ANOVA with post hoc using least significant difference test. Percent decrease in the mean number of worms, eggs, granuloma diameter, and number was calculated from the equation [(control-treated/control)×100]. SPSS computer program (version 16 for Microsoft Windows) was used for data analysis. P-value ≤0.05 was considered significant and<0.01 was considered highly significant.
RESULTS
Anti-SMALDO IgG antibodies level
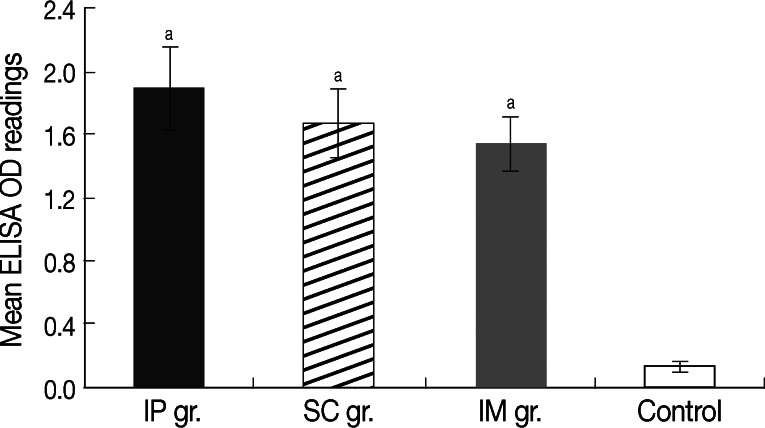
High anti-SMALDO IgG titer was recorded in all groups vaccinated with right-oriented pcDNA3.1/SMALDO (P<0.01) relative to infected control group vaccinated with reverse-oriented pcDNA3.1/SMALDO (Fig. 1). No significant difference was noted between the different vaccinated groups.
Parasitological criteria
Vaccination of mice with SMALDO gene via IP route recorded the highest significant reduction in worm burden (46.2%, P<0.01), number of eggs/g tissue (liver and intestine) (41.7% and 40.2%; P<0.01), number of immature and mature eggs (P<0.01), and increase in the number of dead eggs (P<0.01) compared to their corresponding vector vaccinated infected control group. On the other hand, the SC-vaccinated group showed significant reduction in worm burden (28.9%, P<0.01), number of immature and mature eggs (P<0.01 and <0.05, respectively), and significant increase in the dead eggs (P<0.01), but the number of eggs/g of tissue (liver and intestine) was insignificantly decreased (6.3% and 2.1%, respectively). Finally, IM group recorded insignificant reduction in worm burden (15.1%, P>0.05), number of eggs/g of tissue whether in liver or intestine but it recorded the most significant increase in the number of dead eggs (P<0.01) (Table 1).
Histopathological criteria
All SMALDO DNA vaccinated groups showed a significant reduction in the mean granuloma diameter and number when compared to the vector vaccinated infected control group (P<0.01). IP vaccinated group recorded the smallest granuloma diameter and number (29.2% and 54.7% reduction) followed by SC group (26.6% and 47.8% reduction) and finally IM group (19.5% and 27.4% reduction) (Table 2; Figs. 2, 3). This reduction was significant in both IP and SC groups when compared to IM group. Also, the percentage of dead miracidia inside the eggs was significantly increased in IP and SC groups (84.5±7.5 and 74.2±8.9) compared to vector vaccinated infected controls (31.7±18.0, P<0.01) and IM group (50.1±3.5, P<0.05 and P<0.01, respectively) (Table 2).

Effects of SMALDO-DNA vaccination on different histopathological parameters in different studied groups infected with 80 S. mansoni cercariae and sacrificed at week 8 post-infection

Photomicrograph of liver granulomas of S. mansoni-infected vector-vaccinated control and infected SMALDO vaccinated groups. (A) Vaccinated control group: a large granuloma formed of a central egg with living miracidium surrounded by a large number of eosinophils, neutrophils, and lymphocytes. (B) IP, (C) SC, and (D) IM groups showing small cellular granulomas, formed of an egg in the center surrounded by lymphocytes and few eosinophils and thin collagen fibers (H-E stain, ×200).

Photomicrograph of liver granulomas of S. mansoni-infected vector-vaccinated control and infected SMALDO vaccinated groups.(A) Vaccinated control group: a large fibrocellular granuloma formed of a central egg surrounded by inflammatory cells and deposited collagen fibers. (B) IP, (C) SC, and (D) IM groups showing small cellular granulomas formed of a central egg, surrounded by lymphocytes, histiocytes, fibroblasts, and thin concentric collagen fibers (Masson's trichrome stain, ×200).
Almost all granulomas in the vector-vaccinated control group were large-sized and fibrocellular (90%) consisting of central eggs (most of the eggs inside the granulomas exhibited intact cellular miracidia) surrounded by numerous eosinophils, neutrophils, lymphocytes, few epithelioid cells, macrophages, and concentric collagen fibers. The remaining 10% were large cellular granuloma formed of central eggs (most of the eggs inside the granulomas exhibited living cellular miracidia) surrounded by numerous eosinophils, neutrophils, lymphocytes, few epithelioid cells, macrophages, and scattered collagen fibers. In contrast, most granulomas of IP and SC were small-sized, fibrocellular (60%), and cellular (40%) formed of central eggs where the majority of miracidia were dead surrounded by significantly increased number of lymphocytes (P<0.01), and significantly lower eosinophils (P<0.01), epithelioid cells, and macrophages (P<0.01). A few thin collagen fibers were detected in some early fibrocellular granulomas. In the IM group, 40% of the granulomas were fibrous and no cellular granulomas were recorded (Figs. 2, 3; Tables 2, 3).
With regard to the cellular profile of egg granulomas, the percentages of lymphocytes were significantly higher (P<0.01) in both IP (58.7±7.42) and SC (55.7±7.6) vaccinated groups compared to both control (36.4±5.3, P<0.01) and IM (48.3±10.0, P<0.05) vaccinated groups, whereas the percentages of esinophils were greater (P<0.01) in the control group than in all SMALDO vaccinated groups (Table 3).
DISCUSSION
Reduction in worm numbers is the standard for anti-schistosome vaccine development but, as schistosome eggs are responsible for transmission and pathology, vaccine targeted towards parasite fecundity, egg viability and improving the histopathological status of liver also appears to be relevant [10]. DNA vaccination, mainly based on delivering DNA constructs encoding and expressing specific immunogens into animal cells, has promising results in protection of animals and humans against pathogenic microorganisms [26].
In this study, we tried to evoke an optimum protection against S. mansoni through DNA vaccination with pcDNA3.1/V5-His© TOPO plasmid harboring the full-length of SMALDO using different routes of injection. The gene was cloned using modified primers. The forward primer included Kozak sequence for efficient translation initiation [27] and enhanced in vivo expression of the protein encoded by the pcDNA3.1-SMALDO construct in mammalian cells [11], while the reverse primer was designed to include stop codons at the end of the ORF to express only the native protein.
Results showed that pcDNA3.1-SMALDO DNA-vaccinated mice immunized via the different routes of injections had developed significantly higher titers of anti-SMALDO IgG antibodies when compared with control group vaccinated with construct harboring reverse-oriented SMALDO gene. This revealed the expression of SMLADO polypeptide in the host tissue cells and the development of at least specific humoral immunity towards SMALDO.
In our study, changing the route of DNA vaccination resulted in different levels of protection. IP vaccination gave the maximal protective effects against S. mansoni infection (46.2%) followed by SC (28.9%). This protection was accompanied by a significant reduction in the number of hepatic and intestinal eggs (41.7% and 40.2%) in IP vaccination only with significant increase in dead eggs. Although very little is known about DNA vaccination via the IP route, Cherif et al. [28] vaccinated mice with nanoparticles coated with a plasmid encoding Plasmodium yoelii MSP1-C-terminus protein via IP and IV routes and reported that 100% survival against lethal infection was achieved. To the contrary, IM vaccination had no protective effects but it recorded the highest increase in the number of dead eggs which could be resulted from directed immune responses against the living miracidia inside the eggs but not the worms. In our laboratory, Ahmed et al. [14] reported that IM vaccination with SM21.7 DNA construct, another S. mansoni molecule, induced a significant protection (41.5%) against S. mansoni infection and reduced the number of hepatic and intestinal eggs by 62% and 67%. In our study, increasing the number of dead eggs in oogram pattern in all studied groups compared to the control group could be attributed to both the stage-specific immune response elicited in each vaccination route and also to the anti-egg viability potential of SMALDO DNA which renders the egg death on the expense of immature ones.
The route of DNA vaccine delivery has an impact on the efficiency of transfection of DNA into cells as well as types, location of cells transfected, and antigen presentation, and consequently on the nature of the immune response. IM injection transforms mainly myocytes which are not professional antigen presenting cells (APCs) and lack major histocompatibility complex (MHC) II expression. Myocytes can prime T lymphocytes indirectly through cross-presentation of the produced extracellular antigen by dendritic cells that presumably migrate to the site of DNA inoculation. On the other hand, IP and SC injection led to delivery of DNA to specialized lymphoid tissues and direct transfection of professional APCs; splenic monocytes and macrophages in IP and Langerhans and dendritic cells which migrate rapidly to regional lymph nodes in SC. Consequently, IP and SC routes would be more efficient in eliciting potent B- and T-cell responses. However, in SC injection, the injected vector is readily contained in the subcutaneous tissue, with a limited capacity to spread to large populations of the professional APCs [26,29]. Considering the specific antigen presentation properties of target protein, these facts together could explain the enhanced protective efficacy noticed in IP-DNA vaccinated mice in our study followed by SC compared with IM vaccination.
In this study, the level of protection achieved by IP SMALDO DNA vaccination using only the pcDNA3.1-SMALDO construct is significantly higher than that achieved by Marques et al. [9] who used S. mansoni FBP aldolase recombinant protein. Although they got apparently higher level of protection (57%) than that recorded in this study (46.2%), their recombinant FBP aldolase protein was fused to GST which by itself was able to induce greater than 25% protection. Also, they used aluminum hydroxide adjuvant and Propionibacterium acnes protein to stimulate more potent cell-mediated immunity.
The high level of protection achieved using SMALDO DNA vaccination compared to vaccination with its recombinant protein can be assigned to the ability of DNA vaccination to express the native parasite protein antigens in situ for more efficient recognition by B cells and presentation by MHC class I and II systems to efficiently prime helper and cytotoxic T cells. Also, APCs are activated by the bacterially-derived plasmid vector or its CpG motifs. Targeting DNA to dendritic cells using alpha virus vectors or enhancing its uptake by it could highly increase the protection level [26,30].
Also, in our study, IP and SC SMALDO DNA vaccination induced significant reduction in granuloma size and number (P<0.01) which was more significant than that achieved using the recombinant FBP aldolase protein (P<0.05) [12]. These reductions were associated with 40% reduction in fibrocellular granuloma and a significant increase in dead miracidia (P<0.01) in the IP and SC groups, which would significantly reduce granuloma-associated pathological changes [31].
Wynn et al. [32] attributed granuloma down regulation to increase in Th1 cytokines and decrease in Th2 cytokines. In this model, IFN-γ, IL-12, and TNF-α cytokines are required to suppress type 2 responses and maintain reduced liver pathology [33]. Our data have shown a highly significant reduction (P<0.01) in eosinophils, which plays an important role in induction of the Th2 response by providing IL-4 [34]. Harrop et al. [11] demonstrated that recombinant S. mansoni FBP aldolase protein induced significantly high levels of IFN-γ production in mice vaccinated with irradiated cercariae. Considering the Th1 stimulatory effects of the CpG motifs in the DNA vaccine plasmid backbone [26], the enhanced granuloma modulation by SMALDO DNA vaccination may be due to Th1 polarization.
Using IL-12 and IL-18 as adjuvants could induce stronger Th1 response and increase the protective and anti-pathology effects of SMALDO DNA vaccination as shown previously in an attenuated cercariae model and schistosome DNA vaccination [35]. In S. japonicum, co-injection with vector-encoding murine IL-18 enhanced the protective efficacy and Th1 response of fatty acid binding protein DNA vaccination [12]. DNA vaccination using another carbohydrate metabolism-related enzyme, S. japonicum triose phosphate isomerase, has been optimized using codon optimization and electroporation to achieve 50% worm reduction and enhanced Th1 responses [13]. Consequently, using a cocktail of DNA sequences of SMALDO with other S. mansoni genes [15] in addition to already mentioned adjuvants and cytokines could significantly increase the protective efficacy of SMALDO DNA vaccination above the levels achieved here.
In conclusion, our data demonstrated that changing the route of DNA vaccination significantly enhances the protective and anti-pathological capacity of the SMALDO DNA vaccination. SMALDO DNA vaccination via intraperitoneal route is a promising protective and anti-pathology vaccine candidate.
ACKNOWLEDGMENT
This work is supported by Theodore Bilharz Research Institute project No. 74 C, Biochemistry Department (Prof. Dr. Mohamed Aly Saber).