Effect of Ketoconazole, a Cytochrome P450 Inhibitor, on the Efficacy of Quinine and Halofantrine against Schistosoma mansoni in Mice
Article information
Abstract
The fear that schistosomes will become resistant to praziquantel (PZQ) motivates the search for alternatives to treat schistosomiasis. The antimalarials quinine (QN) and halofantrine (HF) possess moderate antischistosomal properties. The major metabolic pathway of QN and HF is through cytochrome P450 (CYP) 3A4. Accordingly, this study investigates the effects of CYP3A4 inhibitor, ketoconazole (KTZ), on the antischistosomal potential of these quinolines against Schistosoma mansoni infection by evaluating parasitological, histopathological, and biochemical parameters. Mice were classified into 7 groups: uninfected untreated (I), infected untreated (II), infected treated orally with PZQ (1,000 mg/kg) (III), QN (400 mg/kg) (IV), KTZ (10 mg/kg)+QN as group IV (V), HF (400 mg/kg) (VI), and KTZ (as group V)+HF (as group VI) (VII). KTZ plus QN or HF produced more inhibition (P<0.05) in hepatic CYP450 (85.7% and 83.8%) and CYT b5 (75.5% and 73.5%) activities, respectively, than in groups treated with QN or HF alone. This was accompanied with more reduction in female (89.0% and 79.3%), total worms (81.4% and 70.3%), and eggs burden (hepatic; 83.8%, 66.0% and intestinal; 68%, 64.5%), respectively, and encountering the granulomatous reaction to parasite eggs trapped in the liver. QN and HF significantly (P<0.05) elevated malondialdehyde levels when used alone or with KTZ. Meanwhile, KTZ plus QN or HF restored serum levels of ALT, albumin, and reduced hepatic glutathione (KTZ+HF) to their control values. KTZ enhanced the therapeutic antischistosomal potential of QN and HF over each drug alone. Moreover, the effect of KTZ+QN was more evident than KTZ+HF.
INTRODUCTION
Schistosomiasis is a chronic and debilitating disease, caused by the blood-dwelling flukes of the genus Schistosoma that exacerbates poverty [1,2]. Although close to 800 million individuals are at risk of contracting the disease and over 200 million people are thought to be infected, schistosomiasis is often neglected [3]. The global burden of schistosomiasis has been estimated at 1.7 to 4.5 million disability adjusted life years (DALYs), but even the highest estimate might be an underestimation of the true burden [1,4].
Praziquantel (PZQ) is the only widely used drug for treatment and control of this parasitic infection. Although no clinically relevant resistance to praziquantel has been described to date, development of drug-resistance remains a growing threat, particularly in view of mounting PZQ pressure [5]. Accordingly, in light of emerging resistance against PZQ, and in spite of the challenges associated with developing new antischistosomal drugs, alternatives to PZQ should be identified. There is no dedicated drug discovery and development program pursued for schistosomiasis, either by the pharmaceutical industry or through public-private partnerships. However, despite the paucity of concerted efforts to develop novel antischistosomal drugs, a number of compounds with promising antischistosomal properties have been identified, such as the synthetic trioxolanes [6], the cysteine protease inhibitor K11777 [7], alkylaminoalkanethiosulfuric acids [8], PZQ analogs [9], and the oxadiazoles [10].
Quinine (QN), a quinolone methanol, is the main alkaloid of Cinchona species [11]. Quinine has been used clinically in parenteral treatment of severe malaria and oral treatment of resistant falciparum malaria [12]. Quinine is a fast-acting drug with a short elimination half-life that has been recommended for the treatment of uncomplicated malaria in pregnant women and for drug-resistant malaria for almost 400 years [13]. Quinine is eliminated mainly by hepatic metabolism through cytochrome P450 (CYP) 3A4 with very little (<15%) excreted unchanged in urine [14]. Seven metabolites have been identified with 3-hydroxyquinine (3-HQN) being the major metabolite [15]. Halofantrine (HF), a phenanthrenemethanol derivative of aminoalcohol, was first marketed in 1988. It was used for oral treatment of uncomplicated chloroquine- and multidrug-resistant Plasmodium falciparum malaria in both adults and children [16,17]. Halofantrine is metabolized into N-debutylhalofantrine (HFM) essentially by CYP3A4. Maximal HF concentrations of plasma are reached at 2 µM and its bioavailability is very weak and erratic [17], and hepatic clearance represents a large part of total clearance.
Interestingly, the artemisinins (artemether and artesunate), which are essential components of malaria treatment and control [18], also possess antischistosomal properties [19-21]. Recently, the antischistosomal properties in vitro and in the mouse model of several quinoline antimalarials, of which QN, HF, and mefloquine, with the highest worm reductions observed with mefloquine (>80%) have been reported [22-24]. Meanwhile, Keiser et al. [22] supposed that the relatively lower worm reductions for QN (55%) and HF (51%) and other quinolines could be due to their reduced bioavailability.
Ketoconazole (KTZ), an imidazole derivative, is an orally active, broad-spectrum systemic antifungal agent extensively used in veterinary medicine. Ketoconazole is well known to potently inhibit CYP3A4 activity in human liver microsomes [25]. Since the major metabolic pathway of both QN and HF is through CYP3A4 subfamily [26,27], inhibition of the isoenzyme by KTZ could account for the increased plasma QN and HF levels. In the present study, and in the light of the above-mentioned data, we examined the inhibitory effects of KTZ on the antischistosomal potential of QN and HF against adult S. mansoni infection in mice, by evaluating parasitological, histopathological, and biochemical parameters.
MATERIALS AND METHODS
Chemicals and treatments
Bovine serum albumin (BSA), Tween-80, ethanol, 5,5'-dithiobis (2-nitrobenzoic acid) [DTNB, (Ellman's reagent)], ethylene diamine tetraacetic acid (EDTA), nicotinamide adenine dinucleotide phosphate reduced (NADPH), nitro blue tetrazolium (NBT), phenazine methosulphate (PMT), potassium dihydrogen phosphate (KH2PO4), reduced glutathione (GSH), sodium dithionite, trichloroacetic acid (TCA), and thiobarbituric acid TBA were purchased from Sigma-Aldrich Chemical Co. (St. Louis, Missouri, USA). Ketoconazole tablets (Nizoral®; JASSEN-CILAG SpA., Italy, for Janssen Pharmaceutica N.V., Belgium) was given in a dose of 10 mg/kg/day prepared in ultrapure water containing 0.01 M hydrochloric acid for 3 consecutive days (on the 47th, 48th and 49th day post-infection) before QN or HF administration. Quinine tablets (Quinine Sulphate®; SmithKline Beecham Pharmaceuticals, Bentford, UK) and halofantrine tablets as halofantrine-HCl (HALFAN®; GlaxoSmithKline, Mayenne, France) were given in a single oral dose of 400 mg/kg as a fresh suspension in 7% (v/v) Tween-80 and 3% (v/v) ethanol. Treatment with either QN or HF started 2 hr after the last dose of KTZ. Praziquantel tablets (Distocide®, EIPICO, El-Asher Men Ramadan, Egypt) were given orally as a suspension in 2% cremophore-El (Sigma-Aldrich) in a dose of 1,000 mg/kg divided equally on 2 consecutive days at day 49 post-infection (PI).
Animals and infection
A total of 56 male CD1 Swiss albino mice, average weight 20±2 g, were bred and maintained at the Schistosome Biology Supply Center (SBSC) of Theodor Bilharz Research Institute (TBRI), Giza, Egypt. Animals were housed in a controlled temperature and light environment, and were given water and commercial chow ad libitum (Tanta Company for Oils and Soap, Tanta, Egypt). After approval by the Institutional Review Board (IRB) of TBRI, the animal experiments were conducted according to the international ethical guidelines at the animal unit. Cercariae of S. mansoni (Egyptian strain) were obtained from infected intermediate host snails maintained at SBSC. Animals were infected with 80±10 cercariae/mouse using the body immersion technique [28].
Animal grouping
Mice were grouped as follows: uninfected untreated control (I), infected untreated (only received drug vehicles) (II), infected and treated with PZQ (III), infected and treated with QN (IV), infected and treated with both KTZ and QN (V), infected and treated with HF (VI), and infected and treated with both KTZ and HF (VII).
Mice were killed by rapid decapitation at day 63 PI (day 14 after treatment). After decapitation, blood was collected and sera were separated by centrifugation at 1,850 g for 10 min and stored frozen at -70℃ pending assay.
Assessment of parasitological criteria
After killing, hepatic and portomesenteric vessels of mice were perfused according to Duvall and De Witt [29], with no resort to general anesthesia, for worm recovery and subsequent counting. Immediately after perfusion, the liver was isolated, chilled on ice and divided into 2 unequal pieces. The small piece of the liver with the middle part of the small intestine was used for determining the number of eggs per gram of liver or intestinal tissues [30], while the other piece of the liver was used for hepatic enzyme assessment. The oogram pattern of the different egg developmental stages in small intestine tissues was also examined [31], in which eggs at different stages of maturity were identified (in groups I-IV) according to the size of the embryo and were counted. In addition, mature eggs containing fully developed miracidia and dead eggs (granular, dark, and semitransparent) were also counted in 3 fragments of the small intestine and the mean number of each stage was calculated.
Histopathology and granuloma measurement
Pieces of the liver recovered from mice were fixed in 10% neutral buffered formalin and processed to paraffin blocks. Sections (4 µm thick) were cut into 250 µm in apart from the proceeding sections to avoid measuring the same granuloma. Five paraffin liver sections were prepared from each animal and stained with hematoxylin and eosin (H-E) and Masson trichrome stains to examine the histopathological changes and for measurement of hepatic granuloma diameters (30/mouse). Measurements of the granulomas were conducted on non-contiguous granulomas, each containing a single egg (with intact or degenerated miracidia), using an ocular micrometer. The mean diameter of each granuloma was calculated by measuring 2 diameters of the lesion at right angles to each other [32]. Egg viability was assessed microscopically in the same liver sections and cell composition of granulomas was also investigated.
Preparation of liver homogenates
Liver tissue was homogenized in 4 volumes (w/v) of ice-cold 0.1 M potassium phosphate buffer (pH 7.4) containing 1 mM EDTA, followed by sequential centrifugation in a Christ cooling centrifuge at 4℃ and 600 g for 10 min and then at 10,000 g for 20 min. The first half of the supernatant was collected and kept at -80℃ for subsequent analysis for determination of liver content of GSH, superoxide dismutase (SOD), and lipid peroxidation as expressed by malondialdehyde formation. The second half of the supernatant was subjected to ultracentrifugation at 105,000 g for 60 min at 4℃ in a Sorval ultracentrifuge to yield the microsomal pellet which was resuspended in 0.1 M potassium phosphate buffer (pH 7.4) and stored at -80℃ [33] until assay of the cytochrome P-450 (CYP450) and cytochrome b5 (CYT b5) activities.
Glutathione (GSH) assay
The level of GSH was determined in the liver homogenate according to the method of Ellman [34]. A 0.5-ml aliquot of the homogenate was added to a tube with 0.5 ml of 10% TCA. The tubes were centrifuged at 3,000 g for 10 min. A 0.2-ml aliquot of the resulting supernatant was added to a tube containing 5 ml of 0.1 M phosphate buffer and 0.1 ml of DTNB (Ellman's reagent), and the absorbance was measured at 412 nm. With the help of the standard curve drawn using gradual concentrations of a standard GSH, the content of GSH in the liver homogenates of the experimental animals were calculated.
Superoxide dismutase (SOD) assay
SOD activity was assayed spectrophotometrically at 560 nm by the procedure of Winterbourn et al. [35]. The activity of SOD depends on its ability to inhibit phenazine methosulphate (PMT)-mediated reaction of nitroblue tetrazolium (NBT) dye. One unit of enzyme activity is defined as the amount of enzyme that causes half-maximal inhibition of NBT reduction. Activity was expressed in µmole/min/g liver.
Assessment of hepatic lipid peroxidation products
Degree of lipid peroxidation in the liver tissue homogenate of mice was determined in terms of thiobarbituric acid reactive substances (TBARS) formation [36]. One-ml of supernatant was mixed with 1-ml of TCA (10% w/v) in a centrifuge tube and centrifuged at 1,850 g for 15 min at room temperature. One-ml of TBA solution (0.67% w/v) was added to 1-ml of supernatant and kept in a boiling water bath for 45 min. Absorbance was read after cooling at 530 nm against a blank containing all the reagents except the tissue homogenate. As 99% of the TBARS is malondialdehyde, TBARS concentrations of the samples were calculated using the extinction coefficient of malondialdehyde, which is 1.56×105 M-1cm-1.
CYP450 and CYT b5 assay
CYP450 and CYT b5 activities were determined spectrophotometrically using a double-beam spectrophotometer (Lambda 3B, Perkin Elmer, Waltham, Massachusetts, USA) by the method of Omura and Sato [37]. In its reduced form, CYP450 binds with carbon monoxide (CO) gas to give a complex with a maximum absorbance in the soret region at 450 nm. Accordingly, it was determined by the CO-difference spectrum of sodium dithionite-reduced samples using an extinction coefficient of 91 mM per Cm for the difference between 450 and 490 nm. Cytochrome b5 was determined by measuring the difference in its spectrum between the sodium dithionite-reduced samples and oxidized form using an extinction coefficient of 185/mM per Cm for the difference between 409 and 424 nm. The specific activities of CYP450 and CYT b5 enzymes were expressed as units of the enzyme content/mg microsomal protein. Microsomal protein concentrations were assayed according to the method of Lowry [38].
Serum enzymes
Concentrations of alanine aminotransferase (ALT) and albumin in the collected sera were estimated using the available commercial kits.
Statistical analysis
Results were analyzed with the use of SPSS software (Version 9.0). Data are expressed as the mean±SD. Comparison between the mean values of different parameters in the studied groups was performed using one-way ANOVA test, with post-hoc using LSD test. The data were considered significant if P-value was≤0.05.
RESULTS
Parasitological studies
Data in Table 1 shows a significant (P<0.05) decrease in males, females, and total number of worms in all treated groups which was to a lower extent for total males in the groups treated with QN, KTZ+HF, and HF compared to infected group. In addition, all treatment regimens except HF-treated group decreased hepatic and intestinal (P<0.05) tissue egg loads significantly accompanied with an increase (P<0.05) in the percentage of dead eggs and a decrease (P<0.05) in the percentage of immature and mature eggs when compared to infected control (Table 2).

Effects of QN or HF alone and in addition to KTZ on the number of male and female worms in S. mansoni-infected mice at week 9 post-infection (i.e., week 2 post-treatment)

Effects of QN or HF alone and in addition to KTZ on the percentage of egg developmental stages and tissue egg load in S. mansoni-infected mice at week 9 post-infection (i.e., week 2 post-treatment)
Treatment with KTZ+QN significantly enhanced the decrease (P<0.05) in female, total number of worms, hepatic and intestinal tissue egg loads, the percentage of immature and mature (P<0.05) eggs and the increase in the percentage of dead eggs (P<0.05) vs the group treated with QN alone. Meanwhile, the treatment with KTZ+HF numerically decreased males, females, and total number of worms, but significantly (P<0.05) enhanced both the reduction in the percentage of immature and mature eggs and intestinal tissue egg load and the increase in the percentage of dead eggs (P<0.05) vs the group treated with HF alone (Tables 1, 2).
Histopathological studies
Liver sections of infected control and treated groups were studied for granuloma size and cellular profiles. All treated groups showed significant reductions (P<0.05) in the mean granuloma diameters when compared to the infected group. KTZ+QN-treated group recorded the smallest granuloma diameter (50.2% reduction) followed by QN-treated group (40.5% reduction) and KTZ+HF-treated group (40.3% reduction) (Table 3; Fig. 1). Moreover, a significant reduction in granuloma diameter (P<0.05) was observed in KTZ+QN-treated group vs QN-treated group. The percentages of degenerated eggs were significantly (P<0.05) higher in all treated groups compared with the infected group. In addition, there was a significant increase (P<0.05) in the percentages of degenerated eggs in KTZ+QN-treated group vs QN-treated group (Table 3).

Effect of QN or HF alone and in addition to KTZ on granuloma diameter and associated histopathological changes in S. mansoni-infected mice 9 weeks post-infection (i.e., 2 weeks post treatment)

Masson's trichrome stained liver sections of (A) S. mansoni-infected untreated mice showing irregularly outlined large fibrocellular granuloma formed of a central egg (with a living miracidium; arrow), surrounded mainly by lymphocytes, eosinophils, and extensive collagen depositions as concentric collagen fibers. (B) PZQ-treated group showing medium circumscribed fibrocellular granuloma (arrow) with eggs starting degeneration. (C, D) QN and KTZ+QN-treated groups, respectively, showing the smallest size and well-demarcated fibrocellular granuloma, formed of central degenerated egg (D; arrow), surrounded by lymphocytes, eosinophils, neutrophils, scattered macrophages, and fewer collagen depositions (C; arrow). (E, F) HF and KTZ+HF-treated groups, respectively, showing small fibrocellular granuloma formed of central degenerated egg (E; arrow), surrounded mainly by lymphocytes, eosinophils, neutrophils, and few concentric collagen fibers (F; arrow) (×200).
At week 9 PI, the inflammatory granulomatous lesions were mainly seen in the hepatic parenchyma and to a lesser extent in the portal tracts. Granulomas were fibrocelluar (85.7%) and cellular (14.3%) consisting of central eggs (most of the eggs inside the granulomas exhibited intact cellular miracidia; Fig. 1A) surrounded by numerous eosinophils, neutrophils, lymphocytes, few epitheloid cells, macrophages, and concentric or scattered collagen fibers. In contrast, granuloms of KTZ+QN, KTZ+HF, QN and HF-treated groups were smaller in size and fibrocellular (50.0%, 62.5%, 75.0%, and 75.0%, respectively) and cellular (50.0%, 37.5%, 25.0%, and 25.0%, respectively) formed of central eggs where the majority of miracidia were dead surrounded by lymphocytes and peripherally located eosinophils, epitheloid cells, and macrophages (Table 3; Fig. 1C-F).
Enzyme assessments
Infection of mice with S. mansoni significantly decreased (P<0.05) tissue GSH content, CYP450, CYT b5, SOD activities, and serum albumin, while increased (P<0.05) tissue malondialdehyde and serum ALT levels compared to uninfected group (Table 4). Treatment with PZQ alone approximately recovered the contents of GSH, the activities of SOD, CYT b5, and the levels of serum ALT and albumin relative to uninfected group. Treatment of S. mansoni-infected mice with QN or HF alone did not produce any significant change in hepatic antioxidant enzymes, CYP450, and CYT b5 activities, or in serum ALT and albumin levels vs those of uninfected group. Meanwhile, treatment with either QN or HF alone and in addition to KTZ elevated malondialdehyde levels. Moreover, the combined treatment regimens with either KTZ+QN or KTZ+HF produced further inhibition in the activities of CYP450 (P<0.05; 85.7% and 83.8%) and of CYT b5 (P<0.05; 75.5% and 73.5%), respectively, relative to groups treated with QN or HF alone (Table 4; Fig. 2). Furthermore, they restored tissue GSH (KTZ+HF) and the serum levels of ALT and albumin (KTZ+QN or KTZ+HF) to their control values.

Effect of QN or HF alone and in addition to KTZ on liver antioxidant capacity, CYP450 and CYT b5 activities and hepatic function in S. mansoni-infected mice 9 weeks post-infection (i.e., 2 weeks post treatment)
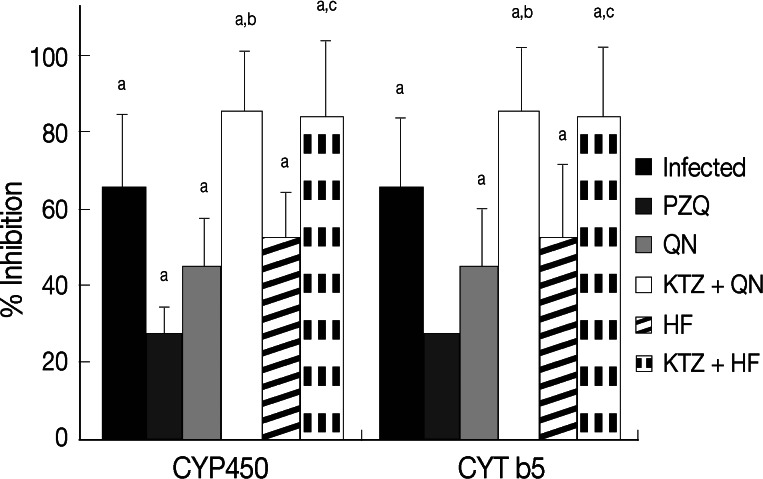
Effect of QN or HF alone and in addition to KTZ on percent inhibitions in hepatic CYP450 and CYT b5 relative to uninfected group in S. mansoni-infected mice at week 9 PI (i.e., week 2 post treatment). No. of animals in each group; 8 mice. Values are presented as the mean±SD. aSignificant difference from uninfected group at P<0.05. bSignificant difference from QN group at P<0.05. cSignificant difference from HF group at P<0.05.
DISCUSSION
To our knowledge, this is the first experimental investigation documenting the inhibitory effects of the CYP3A4 inhibitor KTZ on the antischistosomal potentials of QN and HF against adult Egyptian S. mansoni infection. In the current work, infection of mice with S. mansoni produced a significant decrease in the hepatic activities of CYP450 and CYT b5 (65.3% and 62.1%, respectively). These findings are in agreement with previous investigations [39-41] and are attributed to denaturation of the CYP450 to its inactive form CYP422 as a result of different pathological reactions that follow egg deposition in the liver, including granulomatous, immunologic, and fibrogenic reactions [42]. Moreover, administration of KTZ in the present investigation before QN or HF enhanced the inhibition in the activities of CYP450 (85.7% and 83.8% vs 45.4% and 52.8%) and CYT b5 (75.5% and 73.5% vs 46.0% and 53.7%, respectively) than observed in mice treated with QN or HF alone. It is likely that this pronounced reduction is the augmenting effect of both KTZ and S. mansoni infection. Previous studies reported that KTZ potently inhibit CYP3A4 activity in human liver microsomes and modifies considerably the pharmacokinetics of CYP3A4 substrates such as tacrolimus [25]. Furthermore, KTZ competitively inhibits CYP3A12, a major isoenzyme of the CYP3A subfamily, and at a therapeutic dose, decreases the total body clearance of the CYP3A substrates midazolam [43] in beagle dogs. In addition, KTZ has inhibitory potency for not only CYP3A but also P-glycoprotein (P-gp) in the rat liver [44]. Likely, our work suggests that oral KTZ affected the hepatic CYP3A4 in mice.
In the present study, the efficacy of QN or HF against adult S. mansoni infection following their administration alone or in addition to KTZ was assessed. Administration of QN or HF alone produced significant reductions in males (59.6% and 54.4%, respectively), females (73.9% and 61.8%, respectively) and total number of worms (65.5% and 57.5%, respectively) compared to infected group. In addition, they decreased hepatic, intestinal tissue egg loads, the percentage of immature and mature eggs and increased the percentage of dead eggs. In this context, Xiao et al. [45] found 80.9-90.3% reduction in total worm burden of adult S. japonicum in mice after a single oral dose of 400 mg/kg of mefloquine, quinine, and quinidine, while halofantrine and lumefantrine showed moderate and poor effects with total worm reductions of 67.5% and 38.4%, respectively. On the other hand, Correa Soares et al. [46] recorded that treatment of S. mansoni-infected female Swiss mice with daily intraperitoneal injections of QN (75 mg/kg/day) from the 11th to 17th day after infection caused significant decreases not only in worm burden (39%) but also in female worm burden (40%) and egg production (42%). Moreover, Keiser et al. [22] reported that QN and HF administered orally at a single dose of 400 mg/kg to mice harboring adult S. mansoni (Liberian strain) resulted in total and female worm reductions of 54.7% and 51.7%, and 62.0% and 57.4%, respectively. In the present investigation, it seems that worms of the Egyptian strain of S. mansoni were more susceptible to QN and HF than the Liberian strain.
The detailed mechanism of action of quinoline methanols on schistosomes remains to be investigated. It was demonstrated that the antimalarial chloroquine inhibits the formation of hemozoin (Hz), a heme detoxification aggregate in S. mansoni female homogenates [47]. It is interesting to note that adult female S. mansoni was more affected by QN or HF than male adult S. mansoni. Differences in drug susceptibility between male and female S. mansoni have been reported previously, e.g., following hycanthone treatment [48] and point to a sex-specific interference of the drug with the target, or different drug targets. In addition, it was shown that 4-aminoquinolines interact with "free" heme, hindering its crystallization into Hz. The "free" heme interacts with membranes and exerts severe toxic effects, ultimately killing the parasite through oxidative stress [49]. In this regard, Corrêa Soares et al. [46] reported that interference with heme crystallization in S. mansoni represents an important mechanism by which antimalarial quinoline methanols exerts their antischistosome action. From all of the 3 compounds the authors tested, QN and quinidine (QND) caused the most striking reductions in the Hz content (40-65%) on female worms, which are more susceptible to interference in this process [47]. Hz formation represents the main heme detoxification mechanism in S. mansoni, accounting for more than 50% of total heme content in female worms [50]. Moreover, it was concluded that reduction in total and female worm load, obtained after treatment with QN, QND, and quinacrine (QCR), was positively correlated with the percentage of Hz content reduction in female worms [46]. The present work, together with previous evidence, describes schistosomicidal activity of QN and HF [22,46].
Herein, results revealed that administration of KTZ prior QN or HF significantly enhanced the therapeutic efficacy against S. mansoni infection vs those treated with QN or HF alone. These effects were more pronounced in KTZ+QN-treated group than in KTZ+HF-treated group. In the present study, the enhanced reduction in female and total number of worms in S. mansoni-infected mice treated with KTZ+QN or KTZ+HF was in accordance with the results on the activities of drug-metabolizing enzymes, because mice treated with QN or HF in addition to KTZ showed more inhibition in the activities of CYP450 and CYT b5. Lower metabolism as a result of lower levels of drug-metabolizing enzymes because KTZ possibly leads to increased QN or HF plasma concentrations and to a greater elimination half-life, which in turn increased the length of parasite exposure to the drug.
Schistosome eggs induce liver fibrosis, which is a common pathological process leading to the development of irreversible cirrhosis [51] and inability of the liver to perform its biochemical functions [52]. In the present investigation, the smallest granuloma diameter (50.2% reduction) was recorded in KTZ+QN-treated group followed by QN-treated group (40.5% reduction), KTZ+HF-treated group (40.3% reduction) and PZQ-treated group (32.3%) when compared to the infected group. Moreover, a significant reduction in granuloma diameter was observed in KTZ+QN-treated group vs QN-treated group. Such reductions in granuloma diameter may be attributed to elimination of adult worms by the treatment regimens leading to sustained diminution of egg-induced immunopathology [53] and consequent stopping of further egg deposition [24,54]. The combined treatment regimen of KTZ+QN after S. mansoni infection had an added merit of maximal amelioration of histopathological changes where the liver sections of this group showed remarkably better cellular architecture upon comparing with the S. mansoni-infected untreated and QN-treated groups. Moreover, granuloma in this group showed either partially or almost completely degenerated miracidia beside the minimal surrounding inflammatory reactions. This may be related to either the enhanced killing of the parasite, with marked reduction in egg deposition as evidenced by the parasitological results or because of immunological and anti-inflammatory effects of QN. This is in accordance with Correa Soares et al. [46] who observed that QN treatment promoted remarkable ultrastructural changes in male and female worms, particularly in the gut epithelium and reduced the granulomatous reactions to parasite eggs trapped in the liver. Although nothing is known about the presence or expression of P-glycoprotein (P-gp) in S. mansoni eggs, P-gp has been observed in external layers of the egg shells of the red stomach worm, Haemonchus contortus, a parasitic nematode of ruminants, suggested that P-gp inhibition might contribute to elimination of parasite eggs [55].
Previous studies have reported that schistosomiasis is associated with free radical liberation and disturbance in the cellular antioxidant system [56,57]. The intracellular concentration of reactive oxygen species (ROS) is tightly regulated by multiple defense mechanisms involving ROS scavenging antioxidant enzymes and small antioxidant molecules. In the present study, S. mansoni infection induced a significant decrease in SOD activity, GSH content, serum albumin level, and enhanced lipid peroxidation (malondialdehyde) as well as the serum level of ALT, and these results are in agreement with those previously obtained [20,57-60]. Fernandez-Checa and Kaplowitz [61] reported that this alteration might influence the capability of the liver to provide protection against oxidative damage. The balance between biosynthesis, uptake, oxidation, and export maintains GSH homeostasis at the cellular level. Its reduction in S. mansoni-infected liver tissues is probably related to a reduced synthesis of the tripeptide by the infected liver, which was evidenced by the reduction in serum albumin in this study. It was manifested by increase in malondialdehyde level that lipid peroxidation was enhanced during infection. The decreased activity of SOD in S. mansoni-infected mice may be due to the enhanced lipid peroxidation or accumulation of superoxide radicals and hydrogen peroxide [62].
In the present work, the administration of PZQ alone approximately recovered the contents of GSH, the activities of SOD, and the levels of serum ALT and albumin relative to uninfected group. This could be due to the antischistosomal properties of PZQ as it eradicates parasites, prevents the possible formation of toxic metabolites of S. mansoni worms, abolishes further insults to the liver through interruption of egg deposition, and to its direct effect on mature miracidia within egg granulomas [63] and stopping emission of soluble egg antigens.
Herein, treatment of infected mice with QN or HF alone did not produce any significant change in hepatic antioxidant enzymes or in serum ALT and albumin levels vs those of uninfected group. Similarly, Corrêa Soares et al. [46] reported no changes in malondialdehyde levels in S. mansoni-infected mice treated with QN, which may be attributed to the increased expression of aldehyde dehydrogenase (ADH) gene, which encodes an enzyme involved in detoxification of lipid peroxidation products such as reactive malondialdehyde and hydroxynonenal [64]. On the other hand, Farombi et al. [65,66] reported a decrease in GSH contents and an elevation of malondialdehyde levels upon HF treatment in rat liver microsomes. Moreover, Adaramoye et al. [67] documented that HF treatment significantly reduced GSH and SOD, while increased malondialdehyde in rat liver as well as their serum ALT. Meanwhile, the treatment with either QN or HF alone and in addition to KTZ elevated malondialdehyde levels. Nevertheless, the combined treatment regimens restored serum levels of ALT, albumin, and hepatic GSH (KTZ+HF) to their control values. These results indicate that the liver microsomal lipid peroxidation could be enhanced by quinoline methanols, QN, and HF, and they could have adverse effects on the antioxidant status of the animals. Under such a condition, the need for antioxidants as adjuvant to antimalarials is recommended, and their presence may be crucial to eliminate the products of oxidative reactions.
In conclusion, administration of KTZ, a CYP3A4 inhibitor, with QN or HF produced more inhibition in the activities of CYP450 and CYT b5 that leads to lower metabolism and clearance, which in turn increased the length of parasite exposure to the drug. Accordingly, KTZ enhanced the therapeutic antischistosomal potential of QN and HF over each drug alone. This was evident by increased female, total worm, and egg reductions and healing of hepatic granulomatous lesions, nevertheless, these effects was more obvious in KTZ+QN than in KTZ+HF. QN and HF have adverse effects on the antioxidant status of the animals; therefore, the need for antioxidants as adjuvant to antimalarials is recommended.
ACKNOWLEDGMENT
This work was funded by an internal research project for basic and applied research, a grant from Theodor Bilharz Research Institute.