Clinical and Pathological Characteristics of Intraocular Cysticercosis
Article information
Abstract
This study aimed to explore the clinical, radiological, and pathological characteristics of intraocular cysticercosis due to Taenia solium metacestode infection. Total 8 patients diagnosed with intraocular cysticercosis at the Red Cross Hospital of Yunnan Province, China were examined retrospectively. Patients with clear dioptic media had undergone fundus chromophotography. All patients underwent B ultrasonography of the ocular region (CT) successive scanning of the orbit and cerebral tissues. Parasites were extracted surgically and then examined pathologically. The fundus chromophotography showed a white and condensing scolex package in the vesicle. The B ultrasonic examination showed a vesicle-like echogenic mass in the vitreous chamber, in which the high-level echo spot was the cysticercus scolex. The pathological examinations showed that the vesicle wall exhibited hyaline degeneration, inflammatory cell infiltration, neuroglial fiber, and glial cell proliferation layers from the inside to the outside. The scolex is round and is composed of the outer tissue (the body wall) and the inner furrow tissue; these tissues migrated together. Primordially differentiated sucking discs were found in one case, but no hooklets were found. The inner scolex tissue was folded like a paper flower. The severity of intraocular disease is closely correlated with the pathophysiological processes of the cysticercus worm. Pathological examination of the intraocular lesions can help to evaluate the course of the disease as well as to provide a scientific basis for effective antiparasitic medication.
INTRODUCTION
Taenia solium or the pork tapeworm is one of the most common parasites that live in humans and pigs [1]. Inadequate cooking, eating raw pork, fecal contamination of pasture and water sources, and feeding pigs with poor-quality, contaminated food can cause these tapeworms to proliferate. T. solium eggs liberated from gravid proglottids can infect humans and hatch out in the small intestine followed by parasitizing the subcutaneous tissues, muscles, brain, eyes, and heart, which lead to cysticercosis [2]. Ocular cysticercosis is not uncommon [3].
Yunnan Province lies near the southwestern boundary of China. This province has a high and widespread incidence of cysticercosis as a result of factors such as eating raw meat, less developed production methods, poor living conditions, relatively poor health care, and quarantine evasion [4]. In this study, we retrospectively examined 8 patients who were diagnosed with intraocular cysticercosis and underwent treatment at the Yunnan Red Cross Hospital. The metacestode specimens of T. solium (Cysticercus cellulosae) were extracted surgically for the analyses of clinical manifestations and pathological characteristics of the disease.
CASES AND METHODS
Cases
Between 2000 and 2011, 8 people were treated for intraocular cysticercosis at the Yunnan Red Cross Hospital, Yunnan Province, China. The patients comprised of 6 males and 2 females whose ages ranged from 11 to 56 years old. All patients suffered from the disease in 1 eye during a period of 1 month to 2 years. They are members of Chinese minorities and live near the boundary of China. Among these 8 patients, 7 had raw meat-eating habit, 3 experienced anteocular shadows, 2 experienced impaired vision, and 1 suffered from eye ache. The presence of cysticercus was not detected in 2 patients until intraocular examination was done for cerebral cysticercosis. Cysticerci worms were found in the vitreous chamber of 5 out of 8 patients, whereas 3 out of 8 possessed subretinal infection. The visual light perception of the patients was approximately 0.5.
Fundus photography
Fundus photographies of all patients were recorded. Photos of good quality which showed clear cysticercus images were selected for analysis.
B ultrasonography
B ultrasonography was conducted by using the ODM2000 ophthalmologic B ultrasound equipment (MEDA Co. Ltd, Tianjin, China). The probe frequency was set to 10 MHZ with proper gain control. Six localities were detected routinely to obtain clear acoustic images. Typical images were stored for analysis of the lesion site, shape, internal echo, and ultrasonic wave attenuation.
Cysticerci specimens
This study was conducted in accordance with the declaration of Helsinki and under an approval from the Ethics Committee of Red Cross Hospital of Yunnan Province. Written informed consent was obtained from all participants. Five patients with cysticerci in the vitreous chamber underwent standard pars plana vitrectomy. During surgery, the turbid proliferative vitreous body was cut, and the vesicle wall of the cysticerci was softly clipped by using retina forceps (cysticerci can be taken out wholly through a surgical incision because of its strong deformability). Among the 3 patients with subretinal cysticercosis, 1 had undergone retinal surgery during vitrectomy, whereas the other 2 had undergone sclerochoroidal surgery. The 8 cysticerci were visually observed first and then subjected to pathological examinations. The vesicle was cut open to discharge the fluid and then placed on a microscope slide. The scolex tissue was exposed, hematoxylin-eosin (H-E) stained, and then compressed using the cover slip.
Cysticercus staging
The following stages of cysticerci were identified according to the results of the fundus chromophotography, B ultrasonography, and pathological observations: 1) the coexistence stage, in which only mild inflammatory reactions occur in the fundus tissue, the vesicle wall of the cysticercus is thin, the inside liquid was transparent, and patients did not exhibit any obvious signs or symptoms; this stage might last for several years, 2) the degeneration and death stage, in which the cysticercus degenerated or died, and numerous antigens were released, which lead to peripheral fibroplasias and severe inflammatory reactions of the tissue (such as cataract, intraocular hemorrhage and exudation, and retinal proliferation and detachment), and 3) the calcification stage, in which the parasite was lysed, absorbed, organized, and calcified after death [5].
RESULTS
Clinical characteristics
The clinical characteristics of 8 patients are shown in Table 1. Three lesions were not severe and were at the coexistence stage. The turbidity of the vitreous body was not serious, and the worm was found anterior to the retina. Ophthalmofundoscopy showed a white translucent vesicle floating in the vitreous chamber, and a white and condensing scolex pouch attached to the vesicle. Upon light stimulation, spontaneous peristalsis of the vesicle could be observed, which caused scolex location changes as well as vesicle surface fluctuation (Fig. 1A). Three lesions were severe and were at the degeneration and death stage. Severe vitreous opacity and numerous proliferation strips on the retinal surface and on the subretinal region were observed. Some twisted, dilated blood vessels appeared as occluded white thread-like vessels. Hyperemia and edema of the optic nerve head with an obscure boundary were found (Fig. 1B). The other 2 patients had cataracts, which was why their fundus characteristics were not obtained. Among the 8 patients, 4 suffered from anteocular segment inflammation. No calcified dead parasite was found.
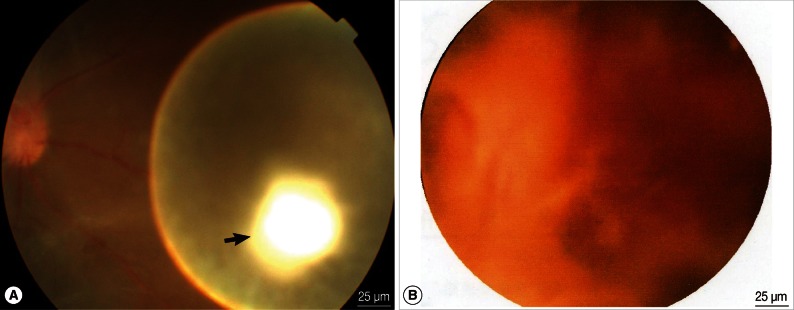
(A) The fundus chromophotograph of an ocular cysticercus in the vitreous body at the coexistence stage. The cysticercus lies anterior to the retina, representing as a white translucent vesicle, and there is a white scolex on the vesicle wall (black arrow). (B) The fundus chromophotograph of a cysticercus in the vitreous body at the degeneration stage. Severe vitreous opacity and many proliferation strips on the retinal surface as well as at the subretinal site are observed. Tortuous dilated blood vessels are seen, with some occluded white thread-like vessels. Hyperemia and edema of the optic nerve head with an obscure boundary are also found.
B ultrasonography revealed a round or oval vesicle-like echogenic mass in the vitreous chamber and a low-level echogenic mass in the vesicle, which indicated that the high-level echogenic spot was the scolex of the cysticercus (Fig. 2A). Through retinal detachment, a high-level echo in the membrane connected to the optic nerve as well as masses of varying echo levels caused by intravitreous bleeding and exudation were detected. For subretinal cysticercus, a strip echo connected to the optic nerve anterior to the cysticercus was detected (Fig. 2B).
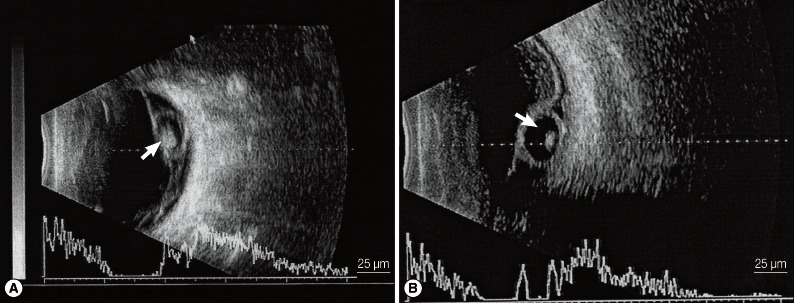
A) A cysticercus in the vitreous body shown by B ultrasonography. A round or oval vesicle-like echogenic mass can be seen in the vitreous body, and the high-level echo spot in it is the scolex (white arrow). B) A cysticercus in the subretinal region shown by B ultrasonography. An echo strip connected to the optic nerve is found anterior to the cysticercus (white arrow).
Pathological characteristics
The vesicle was round or oval and of different sizes, with a diameter that ranges from approximately 5 to 8 mm. The vesicle had a thin translucent wall on the outside and colorless transparent liquid inside. A layer of white membrane was attached to the inner layer of the vesicle, in which a sesame seed-like object, which was actually the scolex tissue, was attached to the thick vesicle wall (Fig. 3A). All scolices of the 8 cysticerci were located inside the vesicle wall without evagination.
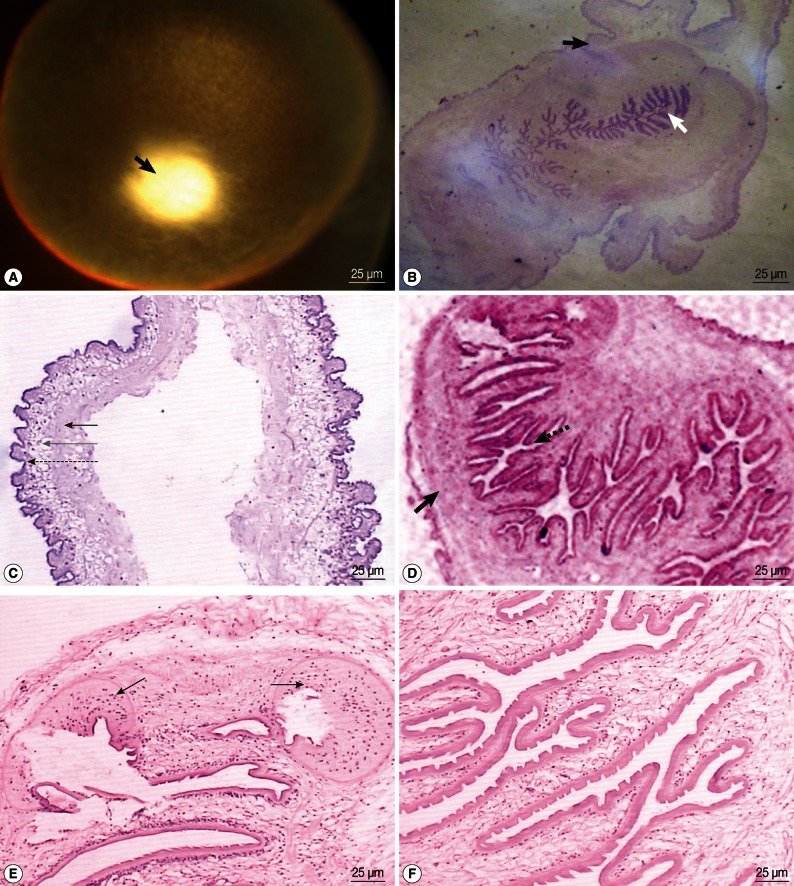
(A) The chromophotograph of a cysticercus in the vitreous body. A round smooth vesicle is seen. It is enveloped by a translucent wall. Colorless transparent liquid is contained in it. White scolex tissue is seen on the wall (black arrow). (B) A cysticercus after HE staining. The peripheral vesicle wall structure is seen (black arrow), and the branch-structured scolex tissue is contained in it (white arrow). (C) The vesicle wall of a cysticercus after HE staining. The inner layer is the hyaline degeneration tissue (black arrow), the middle layer is the inflammatory cell infiltration layer (gray arrow), and the outer layer is the neuroglial fiber and glial cell proliferation layer (dotted arrow). (D) The scolex tissue of a cysticercus after HE staining. The scolex is composed of the outer body wall tissue (black arrow) and the inner furrow tissue (dotted arrow), which migrate with each other. The body wall is not even in thickness. (E) A cysticercus sheet after HE staining. Two round sucking discs are found at the top (black arrows), but no hooklets are seen. (F) A cysticercus sheet after HE staining. The outer layer of the fold is stratum corneum with the Great Wall-like process on it, and the inner layer was porous reticulum with sporadic cells.
After HE staining, vesicle wall tissues with branch-structured scolex tissue could be identified (Fig. 3B). The vesicle wall was characterized by hyaline degeneration, inflammatory cell infiltration, and neuroglial fiber and glial cell proliferation layers from the inside to the outside; however, vascular tissue distribution was not found (Fig. 3C). The scolex was round and composed of the outer tissue (the body wall) and the inner furrow tissue which migrated together (Fig. 3D). The body wall did not have an even thickness. Primordially differentiated sucking discs were found in one case (Fig. 3E), but no hooklets were found. The inner tissue of the scolices was folded in a twisted and rough manner like a paper flower, and had the appearance of an imago body. Fewer folds could be identified when the tissue is closer to the top of the body. Observations under a high-powered microscope showed that the outer layer of the fold, or the stratum corneum, functions as a barrier, and the inner layer was a porous reticulum that contains sporadic cells (Fig. 3F). Based on these pathological characteristics, all patients were diagnosed with cysticercosis, which were proved also by the clinical diagnostic results. The pathological examinations verified that all parasites were in the larval stage. The vitreous body did not exhibit serious turbidity, and the parasite was found anterior to the retina.
DISCUSSION
According to surveys, seropositive rates of cysticercosis in Yunnan Province were 8.2% and 14.1% in 1986 and 1992, respectively. From 2002 to 2004, approximately 800 cysticercosis cases occurred sporadically in this province. Ocular regions such as the vitreous body and subretina are the most common places [5]. The intraocular pathophysiological process of cysticercosis is as follows: an egg develops into a young cysticercus in the small intestine and then escapes through the duodenal wall. It then enters the central retinal artery system through the bloodstream, travels through the central artery directly into the vitreous body, or arrives at the subretinal site after entering the choroid vascular bed through the short posterior ciliary artery, and then enters the vitreous body [6]. After intraocular invasion, toxins and heterologous proteins in the vesicle liquid were released through the wall, which results in local granulomatous inflammation with fibrous tissue proliferation. During the early stage of cysticercosis, retinal edema, exudation, and hemorrhage occur. Although the retinal structure is basically normal, macrophage and eosinophil infiltrations can be observed in each layer. As the lesion develops, a large number of lymphocytes and plasma cells infiltrate the vitreous body, retina, and choroid, thereby leading to severe uveitis, retinal and vitreous proliferation, retinal vascular occlusion, and exudative retinal detachment [7,8]. The retinal tissue directly contiguous to the cysticercus undergoes denatured necrosis and atrophy [9], and complicated cataract and secondary glaucoma may occur. After the vesicle wall ruptures and the parasite dies, a large quantity of toxins are released into the vitreous body, which causes more severe histoclasia and visual impairment [10,11].
The clinical manifestations and iconographical characteristics of intraocular cysticercosis have been extensively reported [12-14]. For the patients with secondary cataracts, intraglomerular bleeding, and severe vitreous exudation and organization, ocular ultrasonography is of great clinical significance. Aside from identifying the morphology and the size of an intraocular abnormal echo zone, this technique determines the anatomic relationship between a cysticercus and the retina to identify whether the parasite is located in the intravitreous body or is subretinal. Based on this judgment, it can be then determined whether to perform sclera incision or vitrectomy. Furthermore, ocular B ultrasonography is easy to perform, non-invasive, and has good repeatability which makes it the preferred examination approach. CT scanning helps to determine whether the central nervous system is affected, especially when patients do not exhibit neurological manifestations. When the brain tissue is affected, several vesicle-like or calcified nodules can be identified. When cerebroventricular obstruction occurs, a hydrocephalus-like change can be detected, and the patients may exhibit symptoms such as headache, epilepsy, coma, and increased intracranial pressure.
To the best of our knowledge, studies about the pathological characteristics of this disease are unreported, which prompted us to conduct the current study. According to the pathological slide observations, the vesicle wall is composed of 3 layers and contains a folded scolex structure. The vesicle wall is responsible for the nourishment and metabolism of the parasite [15], and the scolex structure is the typical pathological manifestation of cysticercus. In the current study, the vesicle walls of all the specimens were thin and transparent, and the scolex tissues could be identified. These results indicate that most of the intraocular cysticercosis cases were caused by the intravitreal cysticercus, and most intraocular lesions were at the coexistence stage, namely that although the patients had varying degrees of fundus vitreous opacity, they had relatively normal retinal structure and thus only exhibited eye shadow or slightly-impaired vision rather than severe infection. When cysticerci stop growing and begin to die, the vesicle wall becomes thickened, calcifies, or shows signs of damage. These changes indicate the transition of the intraocular lesion into the degeneration and death stage. In our study, 3 cases were comparatively severe, but such lesion changes were not observed which indicates that the lesions were in the early stage of degeneration and death, and that obvious changes in the vesicle wall did not occur. This phenomenon is presumably caused by the difference between fundus lesion time and the pathological change time of cysticercus. The scolex tissue is crucial in the cysticercus development. As the scolex develops into an adult, it evaginates out of the vesicle wall, the sucking disk and hooklets achieve maturity, and body wall hyperplasia occurs [16]. After death, the scolex structure disappears and undergoes calcification and fibrosis [17]. In our cases, no further development such as scolex evagination was observed, or in other words, cysticerci were only at the existing form in all the patients, which may indicate that cysticerci do not develop further in the ocular region.
In conclusion, the severity of intraocular disease is closely correlated with the pathophysiological processes of cysticerci; intraocular pathological examinations can help to evaluate the course of the disease as well as to provide a scientific basis for effective antiparasitic medication.
ACKNOWLEDGMENT
This study was supported by the Natural Science Foundation of Yunnan, China (2009ZC175M).
