Seasonal Distribution of Ticks in Four Habitats near the Demilitarized Zone, Gyeonggi-do (Province), Republic of Korea
Article information
Abstract
This study describes the seasonal distribution of larvae, nymph, and adult life stages for 3 species of ixodid ticks collected by tick drag and sweep methods from various habitats in the Republic of Korea (ROK). Grasses less than 0.5 m in height, including herbaceous and crawling vegetation, and deciduous, conifer, and mixed forests with abundant leaf/needle litter were surveyed at United States (US) and ROK operated military training sites and privately owned lands near the demilitarized zone from April-October, 2004 and 2005. Haemaphysalis longicornis Neumann adults and nymphs were more frequently collected from April-August, while those of Haemaphysalis flava Neumann and Ixodes nipponensis Kitaoka and Saito were collected more frequently from April-July and again during October. H. longicornis was the most frequently collected tick in grass habitats (98.9%), while H. flava was more frequently collected in deciduous (60.2%) and conifer (57.4%) forest habitats. While more H. flava (54.1%) were collected in mixed forest habitats than H. longicornis (35.2%), the differences were not significant. I. nipponensis was more frequently collected from conifer (mean 8.8) compared to deciduous (3.2) and mixed (2.4) forests.
INTRODUCTION
Tick-borne disease surveillance is becoming increasingly important as zoonotic tick-borne pathogens are recognized that affect man and wild and domestic animals worldwide [1-3]. In the Republic of Korea (ROK), zoonotic pathogens affecting human health, i.e., spotted fever group (SFG) rickettsia [4], Ehrlichia and Anaplasma spp. [5], Bartonella spp. [6,7], Borrelia spp. [7-9], and tick-borne encephalitis virus [10-12], have been reported from ticks. While ticks collected from birds (migratory bird surveillance) [13], mammals (rodent-borne disease surveillance and large animal surveys) [14,15], and reptiles (wild-life studies) [16] infer tick-host and host-pathogen reservoir relationships, they are often impractical since the live capture of wild animals and birds in their natural habitat is manpower intensive and in some cases requires Biosafety level 3+ (BSL-3+) facilities (i.e., for reservoirs of Hantaan virus) [17-19]. Therefore, other methods, i.e., dragging and sweeping vegetation for questing ticks, are employed for specific environments where associated animal and bird hosts are present. Additionally, unfed larval ticks collected by these methods support evidence for potential transovarial transmission [20].
Previous studies have shown disparities in tick collection methods, as some species are frequently collected from animal hosts, while few are collected when dragging or sweeping vegetation [14]. However, dragging or sweeping vegetation for questing ticks among various habitats frequented by tick hosts provides an estimate of their geographical, habitat, seasonal, and life stage distributions and health-related risks associated with military, agriculture, and recreational activities. The purpose of the present study was to provide estimates of the seasonal abundance and distribution of life stages during periods when ixodid ticks are most active. These data serve to provide information that is necessary for the development of tick-borne disease threat assessments for selected military training sites and privately owned lands as part of the 65th Medical Brigade (MED BDE) tick-borne disease surveillance program.
MATERIALS AND METHODS
Habitats
Tick-borne disease surveillance was conducted at US-ROK operated training sites and privately owned properties in northern Gyeonggi Province near the demilitarized zone (DMZ) that separates North and South Korea, from April-October 2004-2005, as described by Chong et al. [20] (Fig. 1). Habitats surveyed included grasses (<0.5 m), with various proportions of herbaceous and crawling vegetation, and deciduous, conifer (planted groves of pine, fir, larch and cedar), and mixed forests of planted and volunteer deciduous and coniferous trees with abundant leaf and/or needle litter as ground cover and limited understory of patches of grasses, herbaceous vegetation, and shrubs.
Tick collections by dragging and sweeping methods
Tick drags consisted of a 1.5 m long×1.0 m wide flannel cloth attached to a wooden dowel (1.1 m long, 2.0 cm diameter) and a small diameter of 4 m long nylon rope attached to each end of the wooden dowel, as previously described by Daniels et al. [21] and Scoles et al. [22]. The tick sweep was similarly constructed as described by Carroll and Schmidtmann [23], except that it was attached to an aluminum pole bent at a 45° angle where the 1.5 m long×1.0 m wide flannel cloth was attached along the leading edge.
Collection sites included 2 or 4 linear transects that alternated sweep and drag collections conducted concurrently and with collectors/transects separated by approximately 10 m as described by Chong et al. [20]. To reduce "border effect", collection lanes were at least 10 m distant from other habitat types, e.g., adjacent forest habitats and grass habitats. Collections were made by slowly walking and dragging or sweeping the flannel cloth over the vegetation (grasses, herbaceous vegetation, shrubs) or ground cover (i.e., leaf litter), stopping at 5 m intervals, laying the cloth on the ground, and removing the attached ticks using a fine forceps from both sides of the cloth. Nymphs and adults (n=20) were placed in 2 ml cryovials, while larvae (n=100) were placed in 2 ml cryovials containing 100% ethanol. This was repeated until each collector traveled a distance of approximately 100 m.
Ticks were transported to the Entomology Section, 5th Medical Detachment, 168th Multifunctional Medical Battalion (MMB), 65th MED BDE, where they were identified to species and developmental stage under a dissecting microscope using standard keys and current nomenclature [24,25]. The identified specimens were sent to Seoul National University for assay of selected zoonotic pathogens and these data were reported elsewhere [6,26,27].
RESULTS
A total of 19,821 ticks (larvae, nymphs, and adults) belonging to 2 genera and 3 species, H. longicornis (15,020), H. flava (3,889), and I. nipponensis (912), were collected from 4 habitats in northern Gyeonggi-do (Province), ROK, from April through October of 2004 and 2005 (Table 1). Overall, for all stages, H. longicornis [adults 865 (5.7%), nymphs 5,282 (75.8%), and larvae 8,873 (59.1%)] accounted for 75.8% of the 3 species collected from all habitats, followed by H. flava 19.6% [adults 133 (3.4%), nymphs 993(25.5%), and larvae 2,763 (71.1%)] and I. nipponensis 4.6% [adults 35 (3.8%), nymphs 119 (13.1%), and larvae 758 (83.1%)]. Significantly more H. longicornis adults (n=639) and nymphs (n=4,788) were collected from grass habitats and accounted for 61.9% and 74.9%, respectively, of all adults and nymphs collected from that habitat. H. flava adults and nymphs were more frequently collected from conifer forests (433/532; 81.4%) and mixed forests (386/496; 77.8%). Mean numbers of I. nipponensis adults (0.1) and nymphs (0.3-0.8) were similarly collected from all habitats. Significantly more H. longicornis adults and nymphs (ANOVA, F value=10.88, df=3, P<0.001) (mean no. 55.4) were collected from grass habitats, compared to deciduous (8.5), mixed (1.4), and conifer (1.2) forests. Significantly more H. flava (F=15.98, df=3, P<0.001) were collected from conifer (8.0) and mixed (7.4) forest habitats, while few were collected from deciduous forest (3.8) and grass (0.5) habitats. While I. nipponensis nymphs were much less commonly collected from all habitats, significantly more were collected from mixed forests (mean 0.8), while overall, there were no significant differences for adults and nymphs combined from all habitats sampled (F=1.44, df=3, P=0.23).

Monthly mean number (total) larvae, nymph, and adult ixodid ticks collected using tick drag and sweep methods for four habitats at US-ROK operated military training sites and private lands located near the DMZ, Republic of Korea, 2004 and 2005
The seasonal distributions of H. longicornis, H. flava, and I. nipponensis collected by both drag and sweep are shown in Table 1 and Fig. 2. In general, for all habitats, H. longicornis adults were collected more frequently from June-August, while nymphs were most prevalent from April-June. Adult H. flava populations peaked in May, with low numbers collected during the remaining months. Mean numbers of H. flava nymphs peaked in May, declined through September, and then peaked again in October. Mean numbers of I. nipponensis adult and nymph populations peaked during May-June, with fewer numbers collected during April and July-September, with increases in adult numbers during October.
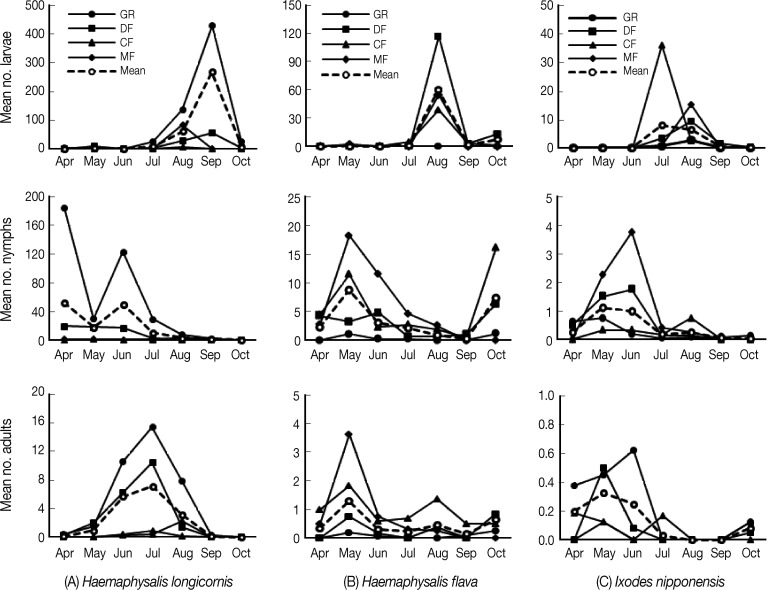
Seasonal distribution of 3 tick species collected from 4 different habitats at US and ROK operated military training sites and installations and private lands located near the DMZ, Republic of Korea, from April through October 2004 and 2005 (GR, grasses and portions of herbaceous and crawling vegetation; DF, deciduous forest; CF, conifer forest; MF, mixed forest).
Large numbers of H. longicornis larvae first appeared in August, peaked in September, and few were collected during October. Large numbers of H. flava larvae were collected only during August that may have resulted in increased numbers of nymphs collected during October. I. nipponensis larval populations peaked in July-August with few collected during the remaining months. While variable patterns were observed for the different habitats, these differences were likely due to host habitat preferences and, in some cases, low numbers collected.
DISCUSSION
The ecology of the ROK following the Korean War consisted of grass and herbaceous covered hills and mountains with occasional scattered trees that provided limited habitat and refuge for medium to large mammals and forest dwelling bird populations. As a result of a national tree planting policy instituted in the 1960s, the hillsides and mountains were populated with planted groves of conifer, deciduous, and later mixed planted and volunteer forests with an understory of associated shrubs and small open areas of grasses and herbaceous vegetation. These ecological changes provided increased habitat, harborage, and an enrichment of flora and fauna provided for the expansion, diversity, and increased numbers of mammals and birds. Concurrently, the geographical dispersion of mammals and birds resulted in the potential for increased tick populations and diversity and prevalence of associated zoonotic tick-borne pathogens that heightens the medical threat for soldiers conducting military exercises and local populations performing agricultural and recreational activities. To evaluate the significance of these changes, a tick-borne disease surveillance program was established to identify the seasonal, geographical, and habitat distributions, relative abundance, and prevalence of tick-associated zoonotic pathogens.
Host and preferred habitat associations are principal for the distribution of ticks. H. longicornis is associated with the large wild and domestic mammals and was collected primarily in habitats consisting of grasses and other herbaceous vegetation frequented by larger herbivores. Hosts of H. flava are associated with small/medium-sized mammals and resident/migratory birds and were collected primarily in forest habitats. I. nipponensis is a 2-host tick with larvae and nymphs feeding on small/medium-sized mammals, while nymphs and adults blood feed on medium/large mammals. Neither sweeping nor dragging methods were highly effective at collecting I. nipponensis [14,20]. However, more than 98.9% of all ticks collected from live captured small mammals (rodents, shrews, and squirrels) in tall grass and herbaceous vegetation in northern Gyeonggi Province was I. nipponensis (mostly larvae, a few nymphs, and no adults), while only one (<0.1%) H. flava, no H. longicornis, and 1.1% Ixodes pomerantzevi Serdyukova were collected [14]. I. pomerantzevi is a nest species and has only been reported from small mammals (squirrels and rodents). Adult and nymph I. nipponensis were more frequently collected in forest habitats, indicating their association with hosts that utilize forest habitats for refuge but perhaps feed or seek prey in open areas with grasses and other herbaceous vegetation.
Unmanaged lands that consist of open areas of tall grasses and herbaceous and crawling vegetation and forested hillsides/mountains harbor large populations of rodents and other small-large domestic (e.g., cats, dogs, cattle, and horses) and wild mammals (e.g., feral cats, raccoon dogs, deer, and wild pigs) and indigenous and migratory birds that serve as hosts for ticks and other ectoparasites [13,14]. Human activities in these areas, whether military or civilian, increases exposure to questing ticks that are potentially infected with tick-borne pathogens. While human activities occur year round, e.g., hiking, construction, agriculture, and military training, they are more common during the months when ticks are active from early spring through late fall.
Tick drag and sweep methods provide estimates of population densities for selected species of ticks and associated disease risks. These data can be used to estimate population densities, identify and determine the prevalence of tick-borne pathogens, determine the potential for transovarial transmission and pathogen maintenance in host and vector populations, and also serve to estimate the potential for transmission of tick-borne pathogens to military and civilian populations as a necessary step for developing disease threat analyses. Tick drag and sweep methods, in addition to collections of ectoparasites from small/large mammals and birds, form the basis of a comprehensive tick-borne disease surveillance program that identifies tick-borne health threats and provides for the development of diseases risk reduction strategies to reduce tick-borne infections among US personnel deployed to the ROK. Finally, in collaboration with US counterparts, local universities, and the Korean Ministry of Health professionals for the identification of ticks and the identification and detection of tick-borne pathogens, these data provides the US military and ROK Ministry of Health with a comprehensive knowledge for endemic tick-borne pathogens and epidemiology of tick-associated diseases in the ROK.
ACKNOWLEDGMENTS
We thank Col. Hee-Choon Sam Lee, Chief, Force Health Protection and Preventive Medicine (FHP&PM), 65th MED BDE, for his dedicated support. We sincerely thank Ms. Suk-Hee Yi, FHP&PM, 65th MED BDE, for providing GIS maps of collection sites and data statistical analysis. We thank Maj. Lisa L. O'Brian and Cpt. Brett W Collier, 5th Medical Detachment Commanders and their staff for assistance with field collections of ticks throughout the study. We also thank Dr. Joel Gaydos, Armed Forces Health Surveillance Center, Global Emerging Infections Surveillance and Response System (AFHSC-GEIS), Silver Spring, MD, for his support throughout this study
Funding for this work was provided through the joint partnership between the AFHSC-GEIS, Silver Spring, MD, the 65th MED BDE, and the National Center for Medical Intelligence, Ft Detrick, MD.
The opinions and assertions contained herein are those of the authors and are not to be construed as official or reflecting the views of the Department of the Army or the Department of Defense.