Prevalence and Genetic Characterization of Toxoplasma gondii in House Sparrows (Passer domesticus) in Lanzhou, China
Article information
Abstract
The prevalence of Toxoplasma gondii infection in birds has epidemiological significance because birds are indeed considered as a good indicator of environmental contamination by T. gondii oocysts. In this study, the prevalence of T. gondii in 313 house sparrows in Lanzhou, northwestern China was assayed by the modified agglutination test (MAT). Antibodies to T. gondii were positive in 39 (12.46%) of 313 samples (MAT titer ≥ 1:5). Tissues of heart, brain, and lung from the 39 seropositive house sparrows were tested for T. gondii DNA, 11 of which were found to be positive for the T. gondii B1 gene by PCR amplification. These positive DNA samples were typed at 9 genetic markers, including 8 nuclear loci, i.e., SAG1, 5'- and 3'-SAG2, alternative SAG2, SAG3, GRA6, L358, PK1, c22-8 and an apicoplast locus Apico. Of them, 4 isolates were genotyped with complete data for all loci, and 2 genotypes (Type II variants; ToxoDB #3 and a new genotype) were identified. These results showed that there is a potential risk for human infection with T. gondii in this region. To our knowledge, this is the first report of T. gondii seroprevalence in house sparrows in China.
Toxoplasma gondii is an important protozoan parasite that infects most species of warm-blooded animals worldwide, including humans and birds [1-3]. It has a complex life cycle in that sexual development occurs only in the intestine of felines, but asexual replication can occur extra-intestinally in many vertebrate hosts. Humans become infected postnatally via ingesting T. gondii tissue cysts from undercooked meat, consuming food or drinking water contaminated with T. gondii oocysts, or by accidentally ingesting oocysts from the environment [4]. Birds as intermediate wild hosts are very important in the circulation of the parasite in wildlife, and T. gondii infection in birds is considered to be epidemiologically significant because of their dietary habit. The house sparrow is frequently commensal with humans and easily adaptable to urban and rural areas [5]. Therefore, the investigation of T. gondii infection in house sparrows is an effective way to estimate environmental contamination with T. gondii oocysts.
The genetic diversity of T. gondii varies in different geographical regions and different hosts. Isolates of T. gondii in South America, for example, are genetically and biologically different from those in North America and Europe [6-13], where the T. gondii population structure is highly clonal and composed mainly of 4 distinct lineages, i.e., Types I, II, III, and 12 [13]. We have previously identified limited genotypes in isolates from humans, cats, pigs, and sheep in China [14-17], but there is little genetic data on T. gondii isolates from wild birds [18]. In the present paper, we report the seroprevalence and genetic characterization of T. gondii isolates from house sparrows in Lanzhou, northwest China.
A total of 313 blood samples were obtained from house sparrows between September and November 2011 in Lanzhou. Blood samples were incubated at 37℃ for 2 hr and then centrifuged at 3,000 g for 5 min to separate the serum samples. The separated serum samples were stored at -20℃ until further analysis.
Antibodies to T. gondii were determined in house sparrow sera by the modified agglutination test (MAT) as described previously [19,20]. Briefly, sera were added to the "U" bottom of 96-well microtiter plates, and diluted 2-fold starting from 1:5 to 1:20. Birds sera with MAT titers of 1:5 or higher were considered positive for T. gondii antibodies based on a previous study [21], those sera with doubtful reactions were re-tested, and positive and negative controls were included in each test.
Heart, brain, and lung tissues from 39 seropositive house sparrows were used for DNA extraction. Genomic DNA was extracted from 5-10 g of heart, brain, or lung tissues by SDS/proteinase K treatment, column-purified (Tiangen™, Beijing, China) and eluted into 50 ml H2O according to the manufacturer's recommendations. A nested PCR targeting the T. gondii B1 gene was performed to detect possible infection with T. gondii [22]. DNA samples giving positive B1 amplification were then used for genetic characterization.
Genetic characterization of T. gondii isolates from sparrows was carried out using the multilocus PCR-RFLP method [16,18,23]. Multiplex PCR-amplified products were 1:1 diluted in sterile, double-distilled water, and then used for nested PCR amplifications with internal primers for each marker, separately. The nested PCR products were digested with restriction enzymes. The restriction fragments were resolved in 2.5% agarose gel, stained by the GoldenView™, and photographed using a gel documentation system (UVP GelDoc-ItTM Imaging System, Cambridge, U.K.).
Thirty nine (12.46%) out of 313 serum samples were detected positive for T. gondii antibodies by MAT (Table 1). However, most of them had low titers, with MAT titers 1:5 in 29 house sparrows, and with 1:10 or higher in 10 house sparrows. Of the 117 DNA samples, 11 were positive for the T. gondii B1 gene by PCR amplification (Fig. 1), including 6 from brains, 3 from lungs, and 2 from hearts. Moreover, among the 11 positive DNA samples, 2 samples were come from the same house sparrow (sample LZS232) but different tissues (brain and heart).

Seroprevalence of Toxoplasma gondii in house sparrows in Lanzhou, northwest China using the modified agglutination test (MAT)
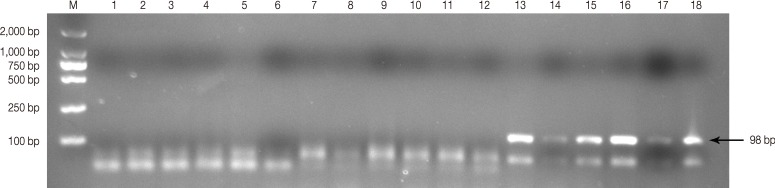
Representative PCR products of Toxoplasma gondii B1 gene amplified from tissue DNA samples of house sparrows. Lanes 1-12 represent negative amplification, and lanes 13-18 represent positive amplification, respectively.
Four DNA samples showed complete genotyping results, 2 from brain (sample LZS232, LZS253), 1 from heart (sample LZS232), and 1 from lung (sample LZS107) (Fig. 2). Due to low DNA concentration, 7 of the 11 positive samples could not be completely genotyped and was therefore not used. Two genotypes (ToxoDB #3 and a new genotype) were identified from the 11 positive samples (Table 2).

PCR-RFLP analysis of Toxoplasma gondii isolates from house sparrows in Lanzhou, China based on 9 different loci. M represents a DNA marker. Lanes 1-10 represent GT1, PTG, CTG, MAS, TgCatCaI, TgCaBr5, LZS253, LZS232 (brain), LZS232 (heart), and LZS107, respectively.
Little is known of the prevalence of T. gondii in sparrows in China. Sparrows could be one of the important species during the cycle of T. gondii transmission. Sparrows especially house sparrows are normally distributed around the human environment. Due to their habit of feeding close to the ground, sparrows, like free-range chickens, are indeed considered as a good indicator of environmental contamination by T. gondii oocysts [4]. More importantly, comparing to chickens, sparrows can transmit T. gondii faster and wider because of their flying ability, hence are more important intermediate hosts of T. gondii than chickens.
Any meat from warm-blooded animals and birds has been traditionally considered a major source of Toxoplasma infection in Western countries [4]. Some local people in this study region like to eat the sparrow meat very much. The risk associated with the type of meat varies among different countries according to local eating habits and according to the T. gondii prevalence in meat-producing animals. We previously reported that 4 of 178 bird DNA samples were positive for the T. gondii B1 gene by PCR amplification. Because serum samples of these 178 birds were not available for serological examination, the data could not indicate the seroprevalence of T. gondii infection in these wild birds in China [18]. Meanwhile, only breast muscle samples were available in that study [18]. Here, we collected heart, brain, and lung tissues for genetic characterization. In the present study, antibodies to T. gondii were examined by MAT, and found in 39 (12.46%) of 313 house sparrows at the cut of 1:5. Although with low titers, house sparrows are easily exposed to T. gondii through the ingestion of food or water contaminated with sporulated oocysts excreted by infected felids. Free-living animals such as wild birds and mammals were considered to be sentinels of environmental spreading of T. gondii oocysts, because of the increasing probability of sharing the same environment with humans [24].
Two genotypes (both are Type II variants) were identified in this study, one of them had a Type I allele at the Apico locus and is considered the Type II variant (ToxoDB #3), which is widely distributed in the world [25-27]. This was the third time that this Type II variant was identified in China, as this genotype was previously reported from sheep in Qinghai Province [17] and wild birds in Xinjiang Uygur Autonomous Region [18]. However, the other strain isolated from sample LZS253 had the Type I allele at the c22-8 locus in addition to the Apico locus, this genotype has not been reported before, and is considered a Type II variant (a new genotype). This is different from previous studies that showed the genotype ToxoDB #9 was predominant in cats and other animals in southern, south-western, and central parts of China [14,17,18,28]. Therefore, the results of the present study and other recent studies indicate that ToxoDB #3 is the major clonal T. gondii genotype circulating in northwestern China [17,18].
To our knowledge, this is the first report of T. gondii seroprevalence in house sparrows in China. The present work also provided new genetic information about T. gondii infection in house sparrows in northwestern China. The prevalence of T. gondii infection in birds has epidemiological significance because infection of the ground-foraging birds like sparrows suggests soil contamination with T. gondii oocysts. These birds are potential reservoirs and good indicators for T. gondii transmission.
ACKNOWLEDGMENTS
The project was supported, in part, by the National Natural Science Foundation of China (no. 31230073, 31172316, 3122 8022, and 31101812), the International Science and Technology Cooperation Project of Gansu Province (Grant no. 1204-WCGA023), the Science Fund for Creative Research Groups of Gansu Province (Grant no. 1210RJIA006) and the National S & T Major Program (Grant no. 2012ZX10004220). The authors thank Dr. J. P. Dubey, Animal Parasitic Diseases Laboratory, Animal and Natural Resources Institute, Beltsville Agricultural Research Center, United States Department of Agriculture, USA for providing the Toxoplasma gondii MAT antigen. Associate Professor Chunlei Su at the Department of Microbiology, the University of Tennessee, Knoxville, USA is gratefully thanked for providing reference T. gondii strains and for constructive comments and suggestions on the draft manuscript.
