Skeletal Manifestations of Hydatid Disease in Serbia: Demographic Distribution, Site Involvement, Radiological Findings, and Complications
Article information
Abstract
Although Serbia is recognized as an endemic country for echinococcosis, no information about precise incidence in humans has been available. The aim of this study was to investigate the skeletal manifestations of hydatid disease in Serbia. This retrospective study was conducted by reviewing the medical database of Institute for Pathology (Faculty of Medicine in Belgrade), a reference institution for bone pathology in Serbia. We reported a total of 41 patients with bone cystic echinococcosis (CE) during the study period. The mean age of 41 patients was 40.9±18.8 years. In 39% of patients, the fracture line was the only visible radiological sign, followed by cyst and tumefaction. The spine was the most commonly involved skeletal site (55.8%), followed by the femur (18.6%), pelvis (13.9%), humerus (7.0%), rib (2.3%), and tibia (2.3%). Pain was the symptom in 41.5% of patients, while some patients demonstrated complications such as paraplegia (22.0%), pathologic fracture (48.8%), and scoliosis (9.8%). The pathological fracture most frequently affected the spine (75.0%) followed by the femur (20.0%) and tibia (5.0%). However, 19.5% of patients didn't develop any complication or symptom. In this study, we showed that bone CE is not uncommon in Serbian population. As reported in the literature, therapy of bone CE is controversial and its results are poor. In order to improve the therapy outcome, early diagnosis, before symptoms and complications occur, can be contributive.
INTRODUCTION
Hydatid disease (echionococcosis; hydatidosis) is a parasitic disease, endemic in many sheep raising countries (Australia, South America, Mediterranean countries, Russia, Middle East, and Central Asia) [1]. Adult cestodes of Echinococcus granulosus inhabit the small intestine of a definitive host (dogs, foxes, and wolves) and produce infective eggs. Through the feces, either cestode segments or free eggs are released into the environment. Intermediate hosts become infected after an oral uptake of the egg. These are ungulates (sheep, goats, pigs, and horses) but in some cases accidental ingestion of eggs, usually shed by dogs, could lead to human cystic echinococcosis (CE). Contaminated food, water, or hand-to-mouth transmission are usual modes of infection in humans [2-4]. So far, 9 genotypes have been described and at least 7 are infective to humans [1].
Once ingested by the intermediate host, in its intestinal tract, parasite eggs transform into the oncosphere. Oncospheres penetrate the intestinal wall, enters the portal vein, and reaches the liver where they usually are trapped and become the hydatid cyst. Liver involvement has been described as the most common location of CE, varying from 59% to 78% of all cases [1,5-8]. Some larvae pass the liver and reach the lungs where they develop into pulmonary hydatidosis. Lung involvement is present in 15-27% of all cases [1,5-8]. Not so often, larvae may pass these filters and form cysts in the brain, skeletal muscles, bones, kidneys, spleen, or other tissues [1,7,8]. Theoretically, they can occur at any site except the teeth, nails, and hairs [9].
The Mediterranean region has always been an endemic area for CE. Epidemiological studies have been conducted in some countries, while in Serbia, Montenegro, BIH, and Albania no information is available about the precise incidence in humans [11]. The aim of this retrospective study was to investigate the skeletal manifestations of hydatid disease in Serbia, it's demographic distribution, site involvement, complications, and bone tissue comorbidities.
MATERIALS AND METHODS
The study was conducted by reviewing the medical database of Institute for Pathology (Faculty of Medicine in Belgrade) which is a reference institution in Serbia for bone pathology. Materials in the study comprised all the cases with histologically verified bone hydatidosis during the period between 1971 and 2010 in the territory of Serbia.
Data obtained from medical records included the age at the moment of diagnosis, sex, localization of hydatid cyst, copresence of hydatid cyst in localizations other than the bone tissue, presence of skeletal complications, bone comorbidities, and diagnosis based on radiographies. In order to standardize the data, the radiological assessment was made on standard radiography, having in mind the length of the study period and diagnostic procedures available for that time. Radiological diagnosis was made according to radiographies made prior to histological verification of echionococcosis. Changes in the bone structure were classified as tumefaction (sharply or unshapely demarcated area of altered bone structure), cysts (sharply demarcated lytic bone lesion with geographical borders), or fractures (in cases where fracture line occured). In some patients, altered bone structure was able to be classified as cyst or tumefaction preceding the pathological fracture, while in other cases, no alteration of the bone structure was radiologically visible aside of the fracture line.
RESULTS
A total of 41 patients with CE were histologically verified. The age of the patients at the time of diagnosis was from 7 to 77 years. The mean age of all patients was 40.9±18.8 years. In order to explore the age distribution of CE, we grouped patients into 5 age categories: I (1-18 years), II (19-36 years), III (37-54 years), IV (55-73 years), and V (>73 years). In group I, the mean age was 13.0±4.2 years, referring to a pediatric population. The mean age of adult patients (more than 18 years old) was 44.7±16.6 years.
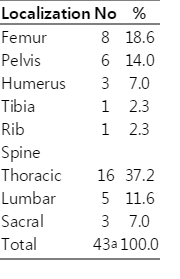
A total of 24 male patients presented 58.5% of all cases, while 17 female patients presented 41.5%. No significant difference between sex was found. Table 1 demonstrates the distribution of skeletal involvement of CE. The spine was the most commonly involved site with the predominance of the thoracic segment. Analysis of standard radiographies revealed that fracture was by far the predominant radiological diagnosis, followed by tumefaction and cyst (Table 2). Pain was the symptom in 41.5% of patients, while some patients demonstrated complications such as paraplegia (22.0%), pathologic fracture (48.8%), and scoliosis (9.8%). The pathological fracture was most frequently found in the spine (75.0%) followed by the femur (20.0%) and tibia (5.0%). However, 19.5% of patients didn't develop any complication or symptom.

Radiological diagnosis based on radiographies made prior to histological verification of bone cystic echinococcosis in Serbian population (1971-2010)
Table 3 demonstrates the distribution of complications and symptoms of spinal hydatid disease. The pathological fracture was the predominant complication, followed by paraplegia. In addition to the bone involvement, 2 patients demonstrated extraskeletal hydatid cysts: 1 in the liver and 1 in the lungs. Bone tissue comorbidities refer to bone tuberculosis in a patient with spinal hydatid disease (2.4%) and to presence of fistula detected in 2 patients (4.9%), 1 with spinal and 1 with pelvic hydatidosis.
DISCUSSION
Serbia is recognized as an endemic country for CE, but so far, no epidemiological study has been conducted [11]. Since echionococcosis is subjected to an obligatory disease for reporting, the data on the number of reported patients were available through the Annual Report on Infectious Diseases on the territory of Republic of Serbia published by Serbian Institute for Public Health, Serbia.
In the study period, a total of 727 patients with CE have been reported in the territory of Serbia. Bone CE was reported in 41 patients, presenting 5.6% of all cases. The frequency was relatively high compared to the reports from other endemic countries, being 0.5-4.0% [1,6,7,10]. This frequency can be explained at least partially by a larger total number of patients [12] due to careless reporting of echionococcosis by physicians, which was even noted in the Official Annual Reports from 1978 to 1989 of Serbian Institute for Public Health, Serbia.
Studies in other endemic countries showed that the prevalence of CE increases with age, resulting that the most of the patients belong to the adult population. The mean age at the moment of diagnosis, including all localizations, was from 33 to 52 years [13-18]. In our study, the mean age of the patients with skeletal manifestation of CE was 40.9±18.8 years, but among the adults the mean age was 44.7±16.6. Higher frequency in aged people could be due to the fact that older people have more chances to be infected with E. granulosus during life, and also, the clinical signs of CE are more probable to appear later in life, as the disease progresses. As it was reported in previous studies [15,19-21], no significant difference between sex was found.
In our study, 12.2% of patients were under 18 years, with the mean age being 13.0±4.2 years. It was consistent with the results of a previous report on the frequency of echinococcosis among children in Serbia [12]. Although boys to girls ratio was 1.5:1.0, no significant difference in the positive rate between sex was found. Some other studies reported slight predominance of boys [16,22,23], which could probably be explained by their outdoor playing, closer contact with animals, and low level of personal hygiene, compared to girls.
Symptoms associated with bone CE are not specific and often missing, making the diagnosis difficult and sometimes overlooked. Our results showed that 19.5% of patients did not report any symptoms and did not develop any complications. These asymptomatic patients are not surprising in consideration that hydatid disease is anywhere in the body and often discovered as an incidental finding [19,24].
Radiological diagnosis of osseous hydatid disease is also difficult because of non-specific findings [7]. Radiography usually reveals single or multiple osteolytic lesions and in some cases shows cortical thinning, osteosclerosis (Fig. 1), and pathological fractures (Fig. 2).

Standard radiography of the left femur (lateral projection) with typical lesion radiologically classified as cyst, accompanied by cortical thinning.

Standard radiography of the right femur (frontal projection) with multiple cystic lesions and associated pathological fracture in the proximal metaphysis (arrow).
Intraosseous foci of hydatid disease predominate in spongious bone tissues and consist of small, separate, and thin-walled cysts. While these cysts expand, they destroy the surrounding trabeculae and can reach a considerable size [25,26]. Due to cyst enlargement and consequent cortical thinning, pathological fractures may occur. In the case of connective tissue proliferation and bone sclerosis, bone CE can radiologically be presented as a tumefaction. In some cases, presentation of CE can be unclear, consisting of mixed radiological signs [26] (Table 2).
Therefore, preoperative diagnosis of skeletal hydatid disease is difficult, and conclusive diagnosis is often made intraoperatively and by histological verification [26-28]. Histological examination of the cyst wall usually reveals an outer chitinous (or fibrous laminar) and an inner germinal layer, surrounded by either granulation tissue or fibrous capsule (Fig. 3). Lamellated cyst walls are hyalinized and collapsed. Protoscolices are often missing [29,30].

Wall of the hydatid cyst showing the laminated membrane with germinal layer and fibrous capsule (H&E stain) (A)×40, (B)×100.
Osseous CE is almost invariably related to primary infection and is not the result of extension from neighboring soft tissue lesions [25]. However, some authors report that around 30-45% of bone hydatidosis is co-present with the hydatid cysts in other localizations [14,31]. We have reported just in 4.9% of cases the presence of hydatid disease in the liver or lungs; however, there is no statement in our database about the radiological surveys for the possible diagnoses of hepatic or pulmonary hydatidosis. Therefore, it is impossible to exclude the presence of secondary cysts without a whole body scan [32].
In patients with bone CE, spine is the most commonly affected site with the frequency of appearance 35-50% [24,29, 33-36]. Our findings (Table 1) are in concordance with the ones reported by other authors, with the exception of the cervical region. Reported results indicate that thoracic segment suffers the most, leading to many complications.
Spinal hydatidosis can be manifested by different clinical features. It is considered to be relatively silent, slowly progressive disease with a latent period of many years [37,38]. Early symptoms appear when the small cyst expands and produces significant mechanical compression on surrounding vital structures. At this stage, intermittent pain can be present as a symptom. As the disease progresses and the cyst enlarges, patient starts suffering from persistent backache, gradually leading to severe neurologic deficit and varying degrees of weakness of limbs [17,26]. Pain was present in 50% of patients with spinal hydatidosis in our study (Table 3) with the frequency of appearance similar to the ones reported by other authors [26,39,40].
Paraplegia is the most serious complication of vertebral hydatidosis appearing in up to 75% of patients with spinal EC [14,38]. This compressive effect of spinal CE was present in 37.5% of patients in our study (Table 3). Paraplegia is a neurological complication, occurring as a result of invasive intradural and extradural growth of the cyst. Cysts invade the spinal canal either by direct compression or by ischemic changes in the spinal cord [17,38]. Radicular pain usually precedes paraparesis by few weeks, progressing rapidly to paraplegia in the thoracic spine, but slowly in the lumbar spine [38]. Our results show that, in some patients, paraplegia can be developed as a compressive effect not just of the cyst, but of the spinal pathological fracture that CE can lead to, as well. All these data indicate that once formed in the spinal column, hydatid cyst is likely to result in paraplegia during the progression of the disease.
In long-term hydatidosis, apart from pain and paraplegia, some cases can result in secondary infections and fistula formation [26,41]. Fistulas were present in 4.9% of patients in our study. Their presence can resolve the disease in the term of surgical treatment, while bacterial superinfection can lead to sterilization of the parasite, limiting the bone destruction [26]. Some authors reported that 30% of bone CE was false diagnosed as Potts disease [24,26]. Interestingly, in our study, 1 male patient was reported with bone CE and tuberculosis super-infection.
Despite CE shows the spinal tropism causing severe complications, the therapy of spinal CE is still poor [27]. The use of anthelmintic drugs is questionable. They are applied in order to retard the recurrences and control the disease but radical surgery is still the keystone [26,42,43]. Data from the literature reported that the recurrence rate of bone CE, even after several operations, varied from 30% to 100% [44]. Moreover, the mortality rate of spinal CE is unacceptably high, varying 14-58% [38].
When CE is affecting long bones, it is generally the metaphyseal region, extending later to the diaphyses and nearby bones [26]. The incidence of long bone involvement in our study (Table 1) was similar to the results reported by other authors [7,34,36]. Pathological fracture is likely to occur, presenting the most severe complication (Fig. 2). Therapy is surgical resection, performed if lesion is segmental, otherwise, amputation or disarticulation are the only solutions [26,39].
Pelvic hydatidosis has also been described in the literature as common. Our results (Table 1) did not differ from the ones obtained by other authors [7,24,34]. The spongy tissue in the pelvis constitutes favorable ground for spreading of hydatid lesions. It's progression is infiltrating and diffuse and may lead to pain, swelling, and stiffness of the hip joint.
The progression of CE in bone tissues is insidious. This disease is hematogenously seeded along the trabeculae and through the medullar canal, leading to extensive bone lesions [13]. Our results reported that these lesions were mostly complicated with pathological fracture (48.8% of all cases).
Pathological fracture is reported to most commonly affect long bones [44]. Our results reported primary involvement of the spine (75%), with the predominance of lumbar over thoracic segment. In patients with bone CE, bone tissue destruction develops through 3 mechanisms; as the consequence of the cystic lesion expansion and its compression on surrounding tissues, ischemic processes through compression and obstruction of nutrient vessels, and cellular processes like osteoclast proliferation around the compressed bone tissue [26]. In the absence of pericyst formation, the wall of the cyst is thin and cannot overcome the rigid bone nature. Therefore, the cyst cannot assume it typical, spherical shape, and it enlarges irregularly along the path of least resistance. In patients with skeletal CE, bone destruction lead to its thinning, extension into soft tissues and, as the worse case, can lead to fracture. Therefore, some authors suggest that all the pathological fractures in endemic countries should be considered as a possible case of CE [45].
ACKNOWLEDGMENT
We thank the Ministry of Science of the Republic of Serbia for supporting this research (project no. 45005).