Abstract
Neutrophils are the predominant inflammatory cells found in vaginal discharges of patients infected with Trichomonas vaginalis. In this study, we examined superoxide anion (O2.-) production by neutrophils activated by T. vaginalis. Human neutrophils produced superoxide anions when stimulated with either a lysate of T. vaginalis, its membrane component (MC), or excretory-secretory product (ESP). To assess the role of trichomonad protease in production of superoxide anions by neutrophils, T. vaginalis lysate, ESP, and MC were each pretreated with a protease inhibitor cocktail before incubation with neutrophils. Superoxide anion production was significantly decreased by this treatment. Trichomonad growth was inhibited by preincubation with supernatants of neutrophils incubated for 3 hr with T. vaginalis lysate. Furthermore, myeloperoxidase (MPO) production by neutrophils was stimulated by live trichomonads. These results indicate that the production of superoxide anions and MPO by neutrophils stimulated with T. vaginalis may be a part of defense mechanisms of neutrophils in trichomoniasis.
-
Key words: Trichomonas vaginalis, neutrophil, superoxide anion, myeloperoxidase, growth inhibition
Trichomonas vaginalis is a sexually-transmitted flagellate with a worldwide distribution [
1,
2]. In the Republic of Korea (=Korea), the prevalence of
T. vaginalis in women in Kangwon-do (from 1988 to 1993) and Gyeonggi-do (from 1996 to 1997) were reported to be 7.6% and 10.4%, respectively [
3,
4]. In men,
T. vaginalis infection can cause urethritis and prostatitis, and
T. vaginalis has been detected by PCR in specimens of Korean patients with chronic urethritis or prostatitis [
5,
6].
Numerous neutrophils have been detected in the vaginal discharge of trichomonal vaginitis patients, and attachment of trichomonads to neutrophils has also been observed [
1]. Superoxide anions (O
2.-) are known to play an important role in the protective mechanisms of neutrophils, but it is unclear whether they generate superoxide anions against
T. vaginalis. When superoxide dismutase and catalase (which can scavenge superoxide anions and hydrogen peroxide, respectively) were added to cocultures of trichomonads and neutrophils, fewer trichomonads were killed than in the absence of these enzymes [
7]. These findings demonstrated the importance of reactive oxygen species (ROS) such as superoxide anions and hydrogen peroxide in killing
T. vaginalis by neutrophils. Various oxidants generated by myeloperoxidase, a heme enzyme released from intracellular granules of neutrophils, are known to play an important role in immune responses to invading pathogens [
8]. In this study, we evaluated the production of superoxide anion and myeloperoxidase by neutrophils stimulated with trichomonads.
The human trichomonad,
T. vaginalis (KT4 isolate), was obtained from the vaginal discharge of a Korean woman with vaginitis [
9]. It was subcultured every 24 hr in screw-capped glass test tubes containing 15 ml of Diamond's trypticase-yeast extract-maltose (TYM) medium [
10]. Lysates, membrane components (MC), and excretory-secretory products (ESP) were prepared as described previously with minor modifications. Briefly,
T. vaginalis were harvested in log phase and washed by centrifugation. They were lysed by 3 bursts at 15 sec intervals from a sonicator (Heat System-Ultrasonics, Farmingdale, New York, USA), then centrifuged at 10,000 g for 30 min. The resulting supernatants were used as
T. vaginalis lysates after filtration through 0.22 µm filters [
11]. To prepare the membrane component of
T. vaginalis, trichomonads were homogenized in Tris-lysis buffer (1 mM EDTA, 1 mM EGTA, 1 mM PMSF) (Sigma; St.Louis, Missouri, USA) with 20 strokes of a homogenizer, and centrifuged at 1,000 g for 10 min. The supernatants were then centrifuged at 50,000 g for 30 min, and the pellets were resuspended in a lysis buffer (1.5%
n-octylglucoside) (Sigma), and sonicated 3 times at 15 sec intervals. After centrifugation at 100,000 g for 30 min, the supernatants were dialyzed (against PBS), filtered and used as MC [
12].
T. vaginalis ESP was prepared by incubating 1×10
7/ml trichomonads in a 12-well plate at 37℃ for 90 min then centrifuging them at 10,000 g for 30 min. The supernatants were filtered and used as ESP [
11].
Neutrophils were isolated from healthy donors by dextran sedimentation and Histopaque 1,077 density gradient fractionation, as previously described with minor modifications. Ten volumes of venous blood containing 5 U/ml heparin were well mixed with 2 volumes of 4.5% dextran T500 (Pharmacia, Uppsala, Sweden). After 30 min sedimentation at 37℃, the supernatant was overlayed on Ficoll-Histopaque 1077 and centrifuged at 385 g, at 4℃ for 30 min. After lysis of any erythrocytes in the sediment with sterile ice-cold distilled water, the isolated neutrophils were diluted in culture medium and kept on ice until use. Cell viability (>99%) was determined by the trypan blue exclusion method. To rule out monocyte contamination, cells were counted with a flow cytometer (BD Bioscience, San Jose, California, USA) after staining with fluorescein isothiocyanate (FITC)-conjugated anti-CD14 antibody (BD Bioscience), and neutrophils were found to make up more than 99.8% of the cells. Superoxide anion was measured on the basis of cytochrome
c (Sigma) reduction [
13]. A 100-µl of
T. vaginalis lysate, ESP, or MC was added to each well of a 96-well plate followed by 100 µl of a solution containing cytochrome
c (12 mg/ml) and neutrophils (4×10
4) in Hanks balanced salt solution (HBSS). After incubation at 37℃ in a 5% CO
2 incubator for 30-180 min, the absorbance of each well was measured at 550 nm with a microplate autoreader (Thermomax; Molecular Devices, California, USA).
Superoxide anion generation was calculated using the extinction coefficient (38.16×103 M-1cm-1) of reduced cytochrome c at 550 nm, and is shown as nmoles of reduced cytochrome c per 1×105 neutrophils. To determine the role of trichomonad protease in superoxide anion generation, each trichomonad product (300 µl/ml of lysate, 200 µg/ml of MC, or 100 µl of ESP) was treated with protease inhibitor cocktail (PIC; Boehringer Mannheim/Roche, Mannheim, Germany) for 30 min at 37℃ before incubation with neutrophils. However, the effect of the generated superoxide anion on growth of T. vaginalis was investigated using neutrophil supernatants as follows: neutrophils (5×104) were incubated for 3 hr with T. vaginalis lysate (300 µg/ml) and the supernatant was collected. Trichomonads (5×105) were incubated with this supernatant for 3 hr, then transferred to TYM medium and incubated for 14 hr after which their numbers were counted by light microscopy, and compared with those of trichomonads incubated with control neutrophil supernatants.
For measurement of myeloperoxidase (MPO) release by neutrophils, neutrophil suspensions (1×107) were put into 6-well plates, then live T. vaginalis (1×106) was added at a ratio of 10:1 (=N:T). The final volumes were adjusted to 3 ml. Culture supernatant was collected after 1-24 hr incubation at 37℃ in a 5% CO2 incubator, and released MPO was determined using an ELISA kit (R&D systems, Minneapolis, Minnesota, USA).
For statistical analysis, results are expressed as the mean±SD of at least 3 independent experiments. The Student's t-test was used to determine the statistical significance. Significance was accepted at a P value of<0.05. The statistics program used was SPSS 12.0K (SPSS Inc., Chicago, Illinois, USA).
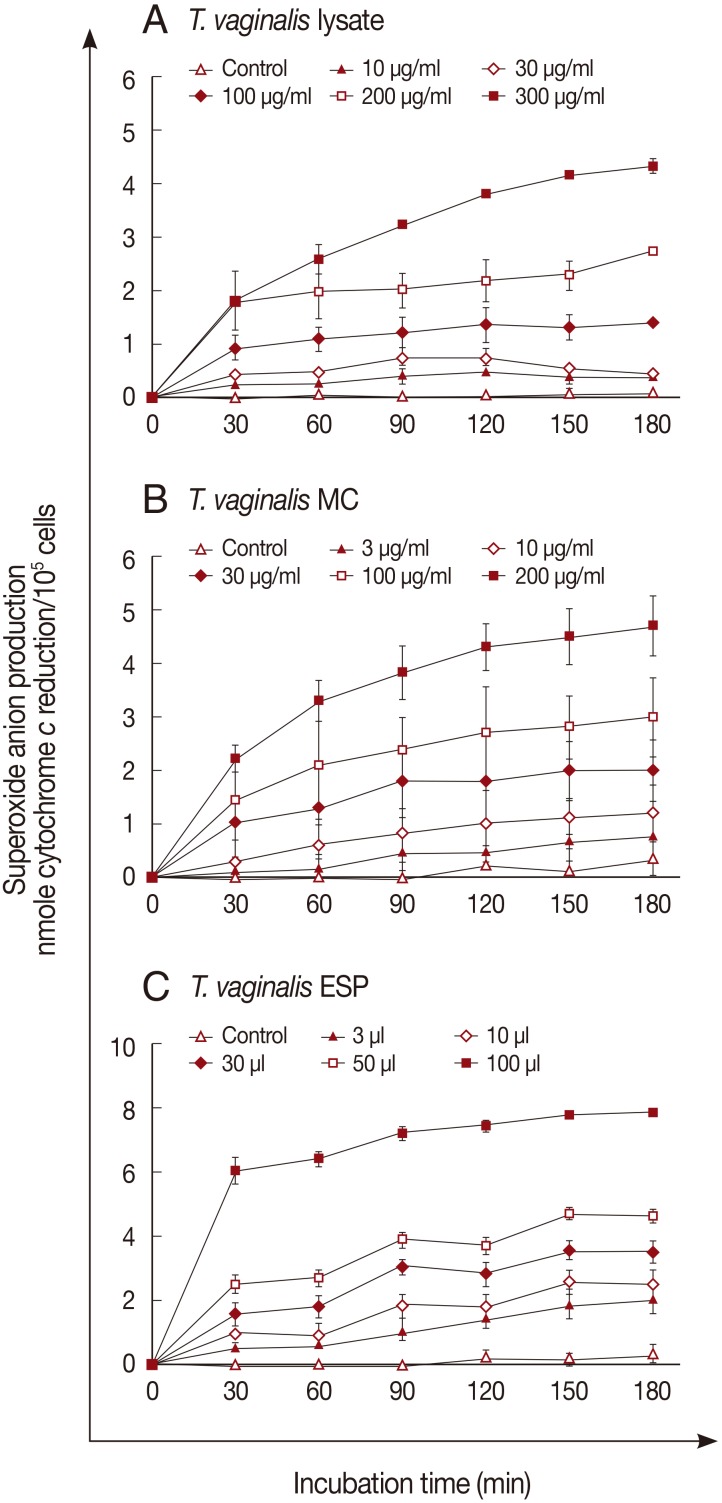
Superoxide anion generation by neutrophils was measured after incubation with the 3 trichomonad products for 3 hr. Superoxide anions were generated in response to
T. vaginalis products in a dose- and time-dependent manner (
Fig. 1). A marked increase of superoxide anion was observed after incubation with 300 µg/ml of
T. vaginalis lysate or 200 µg/ml of MC (
Fig. 1A, B). With ESP (100 µl), superoxide anion generation increased after 30 min incubation (6.0±0.24 nmole), and reached 7.8±0.56 nmole after 3 hr (
Fig. 1C).
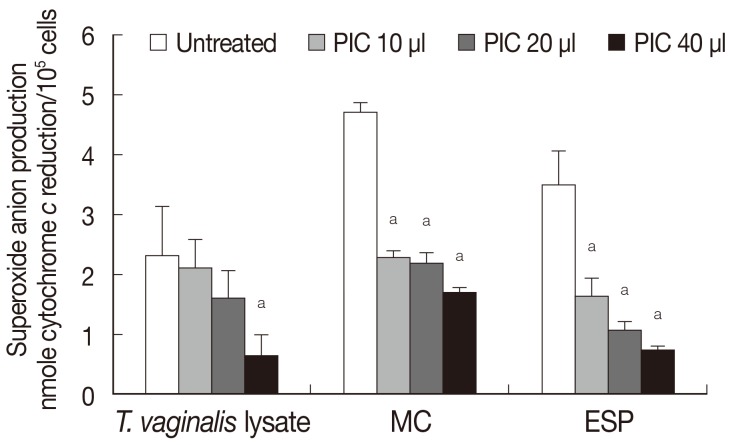
The role of the protease of
T. vaginalis in generating superoxide anion production by neutrophils was determined by adding protease inhibitor (
Fig. 2). Pretreatment with protease inhibitor cocktail (PIC) reduced superoxide anion generation in response to
T. vaginalis lysate in a concentration-dependent manner, and similar results were obtained in the case of ESP and MC (
Fig. 2). Although constituents of the 3 trichomonad products were expected to be different, superoxide anion generation by neutrophils after each trichomonad product treatment showed similar results (
Figs. 1,
2). As shown in
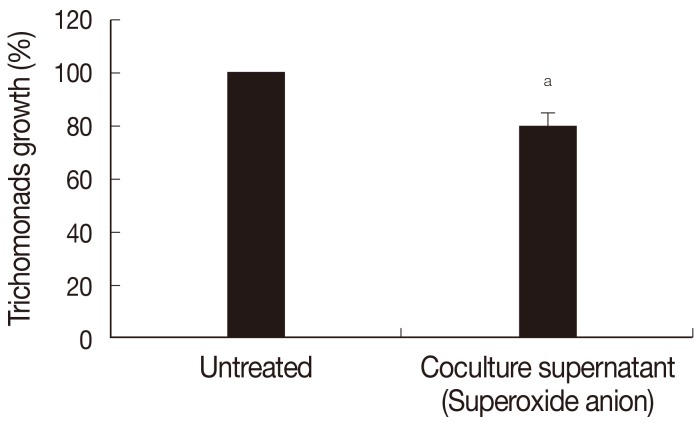
Fig. 3, growth of
T. vaginalis was inhibited by preincubation with supernatants of neutrophils incubated for 3 hr with
T. vaginalis lysate (see Methods part); pretreatment with supernatant reduced the number of trichomonads by 19.8% (
P<0.05) at 14 hr of cultivation.
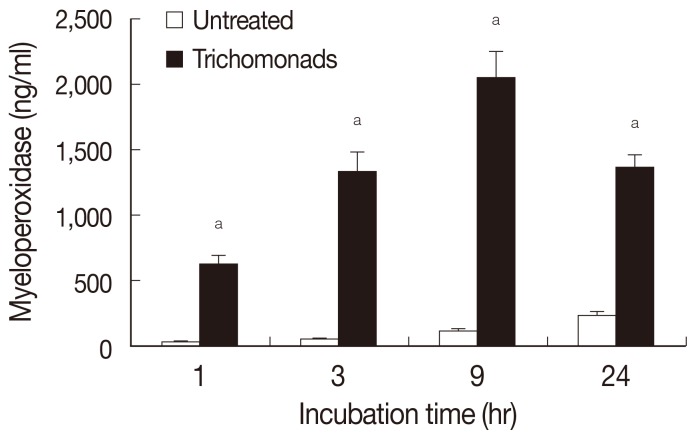
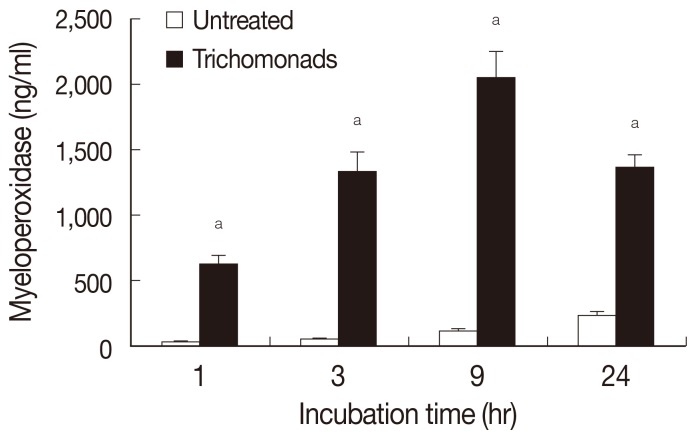
Myeloperoxidase release by neutrophils was determined by ELISA after stimulation with live
T. vaginalis (
Fig. 4). In this experiment, live
T. vaginalis increased MPO after 1 hr of incubation (624±64 ng/ml), and secretion of MPO increased up to 9 hr (2,049±196 ng/ml).
Neutrophils are regarded as one of the key components of the innate immune response, and are involved in the first line of defense against invading pathogens, along with macrophages, eosinophils, and platelets. The microbicidal activities of neutrophils and macrophages are usually classified according to their oxygen dependence. The production of ROS and of reactive nitrogen species (RNS) are regarded as key processes in the oxygen-dependent microbicidal pathway. Reactive oxygen species are involved in various cellular activities, including stimulation of cell proliferation or cell death [
14,
15]. Examples of ROS include the superoxide anion (O
2.-), hydroxyl radical (OH), hydrogen peroxide (H
2O
2) and hypochlorite anion (ClO
-). Superoxide anion generation of neutrophils with some parasite infection, such as cysticercus cellulosae (=the metacestode of
Taenia solium) and
Plasmodium vivax, was previously reported [
16,
17]. Likewise, reactive nitrogen species, such as nitric oxide (NO), nitrogen dioxide (NO
2), and nitrous acid (HNO
2), play important roles in various physiological processes [
18]. However, ROS and RNS also contribute to the oxygen-dependent antimicrobial activities of phagocytic cells. During phagocytosis, oxygen uptake into phagocytes, called the respiratory burst, is increased. After activation of NADPH oxidase in phagosomal membranes, O
2 is reduced to the relatively stable intermediate, superoxide anion which can be bactericidal. Superoxide anion is the precursor of other powerful oxidants such as hydroxyl radicals [
19]. The superoxide anion is related to RNS generation. For example, peroxynitrite (ONOO
-), one of the RNS, is formed by a reaction between superoxide anion and nitric oxide [
20]. Myeloperoxidase is released from intracellular granules of neutrophils and contributes to their microbicidal activity. The generation of various oxidants including hypochlorous acid (HOCl) and hypobromous acid (HOBr) by myeloperoxidase in the presence of superoxide anion is believed to contribute to the antimicrobial activities of neutrophils [
21,
22].
In a previous report, neutrophils were shown to kill
T. vaginalis in aerobic conditions, and the addition of catalase or superoxide dismutase prevented this death [
7]. This suggested that superoxide anion and hydrogen peroxide were important in killing of trichomonads by neutrophils. Moreover, the observation of reduced nitroblue tetrazolium (NBT) precipitates during co-incubation of neutrophils and trichomonads indirectly confirmed that superoxide anion was generated. In our present experiments, we directly measured superoxide anion generation by neutrophils stimulated by various products of trichomonads (
Fig. 1). Our results were in good accord with previous reports done with other parasites [
16,
17]; however, further work is required to understand detailed stimulation mechanism of each trichomonad product and the role of superoxide anion in killing of trichomonads.
Chemorepulsion has been reported when trichomonads and an oxygen metabolite, such as hydrogen peroxide or hypochlorite, are placed on either side of polycarbonate membrane filters [
23]. Migration of
T. vaginalis into the filter was observed when neutrophils were placed alone on the opposite side of a filter. However, the addition of cell-free hydrogen peroxide or hypochlorite inhibited the movement of trichomonads across the filter due to chemorepulsion. Moreover, chemotaxis of neutrophils to trichomonad ESP has been reported [
24]. These observations throw an interesting light on the relationship between host and parasite.
T. vaginalis protease has been shown by electrophoresis to be a typical cysteine protease [
25]. The trichomonad protease is required for adhesion to epithelial cells, and for escape from the host immune system by degradation of human immunoglobulins [
11,
26]. We showed previously that
T. vaginalis protease was involved in IL-8 production by neutrophils [
27]. In the present work, the decreased superoxide anion production by neutrophils incubated with PIC-pretreated
T. vaginalis indicates that the trichomonad protease is also involved in superoxide anion production (
Fig. 2). In addition to PIC, iodoacetic acid (IAA), a cysteine protease inhibitor, and EDTA, a metalloprotease inhibitor, also decreased the production of superoxide anion (data not shown). Further research is needed to define the exact mechanism of action of the protease.
Myeloperoxidase is a heme enzyme found in the intracellular granules of neutrophils and primary lysosomes of monocytes. During the oxidative burst, MPO in activated neutrophils oxidizes chloride ions (Cl) with hydrogen peroxide (H
2O
2) as oxygen donor, and produces hypochlorous acid (HOCl), which phagocytic cells use to kill microbes [
22,
28]. Interestingly, genetically-modified MPO-deficient mice, whose neutrophils lack MPO activity have increased susceptibility to
Candida albicans infection [
29]. MPO-treated
Entamoeba histolytica showed morphological alterations, leading to death [
30]. It has been suggested that killing of
Staphylococcus aureus by neutrophils is caused by myeloperoxidase-dependent production of superoxide anion [
31]. Substantially more frequent severe infection has been noted in MPO-deficient human individuals [
32]. In the present study, 3 different trichomonad preparations triggered the release of superoxide anion and MPO in neutrophils and these products are likely to be directly involved in killing of
T. vaginalis.
In conclusion, the release of superoxide anion and MPO by neutrophils activated by trichomonads appears to be involved in defense mechanisms of human neutrophils against T. vaginalis.
ACKNOWLEDGMENT
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2011-0012374).
References
- 1. Fouts AC, Kraus SJ. Trichomonas vaginalis: reevaluation and its clinical presentation and laboratory diagnosis. J Infect Dis 1993;141:137-143.
- 2. World Health Organization. Prevalence and incidence of selected sexually transmitted infections, Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis, and Trichomonas vaginalis: methods and results used by the WHO to generate 2005 estimates. WHO; 2011.
- 3. Choi KS, Kwon JY, Uh Y, Koo JS, Cha DS, Kim MC. Prevalence of vaginal Trichomanas vaginalis in Kangwon area. Korean J Obstet Gynecol 1996;39:1273-1278.
- 4. Ryu JS, Chung HL, Min DY, Cho YH, Ro YS, Kim SR. Diagnosis of trichomoniasis by polymerase chain reaction. Yonsei Med J 1999;40:56-60.
- 5. Bar-Chama N, Fisch H. Infection and pyospermia in male infertility. World J Urol 1993;11:76-81.
- 6. Lee JJ, Moon HS, Lee TY, Hwang HS, Ahn MH, Ryu JS. PCR for diagnosis of male Trichomonas vaginalis infection with chronic prostatitis and urethritis. Korean J Parasitol 2012;50:157-159.
- 7. Rein MF, Sullivan JA, Mandell GL. Trichomonacidal activity of human polymorphonuclear neutrophils: killing by disruption and fragmentation. J Infect Dis 1980;142:575-585.
- 8. Davies MJ. Myeloperoxidase-derived oxidation: mechanisms of biological damage and its prevention. J Clin Biochem Nutr 2011;48:8-19.
- 9. Ryu JS, Choi HK, Min DY, Ha SE, Ahn MH. Effect of iron on the virulence of Trichomonas vaginalis. J Parasitol 2001;87:457-460.
- 10. Diamond LS. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol 1957;43:488-490.
- 11. Min DY, Ryu JS, Park SY, Shin MH, Cho WY. Degradation of human immunoglobulins and cytotoxicity on HeLa cells by live Trichomonas vaginalis. Korean J Parasitol 1997;35:39-46.
- 12. Shaio MF, Lin PR, Liu JY, Yang KD. Generation of interleukin-8 from human monocytes in response to Trichomonas vaginalis stimulation. Infect Immun 1995;63:3864-3870.
- 13. Shin MH. Excretory-secretory product of Paragonimus westermani newly excysted metacercariae inhibits superoxide production of granulocytes stimulated with IgG. Korean J Parasitol 2000;38:103-106.
- 14. Li PF, Dietz R, von Harsdorf R. Reactive oxygen species induce apoptosis of vascular smooth muscle cell. FEBS Lett 1997;404:249-252.
- 15. Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J 1991;10:2247-2258.
- 16. Chaible LM, Alba-Loureiro TC, Maia AA, Pugine SM, Valle CR, Pithon-Curi TC, Curi R, De Melo MP. Effect of cysticercus cellulosae on neutrophil function and death. Vet Parasitol 2005;127:121-129.
- 17. Leoratti FM, Trevelin SC, Cunha FQ, Rocha BC, Costa PA, Gravina HD, Tada MS, Pereira DB, Golenbock DT, Antonelli LR, Gazzinelli RT. Neutrophil paralysis in Plasmodium vivax malaria. PLoS Negl Trop Dis 2012;6:e1710.
- 18. McQuaid KE, Keenan AK. Endothelial barrier dysfunction and oxidative stress: roles for nitric oxide? Exp Physiol 1997;82:369-376.
- 19. Candeias LP, Patel KB, Stratford MR, Wardman P. Free hydroxyl radicals are formed on reaction between the neutrophil-derived species superoxide anion and hypochlorous acid. FEBS Lett 1993;333:151-153.
- 20. Zhu L, Gunn C, Beckman JS. Bactericidal activity of peroxynitrite. Arch Biochem Biophys 1992;298:452-457.
- 21. Kettle AJ, Winterbourn CC. Superoxide enhances hypochlorous acid production by stimulated human neutrophils. Biochim Biophys Acta 1990;1052:379-385.
- 22. Winterbourn CC. Biological reactivity and biomarkers of the neutrophil oxidant, hypochlorous acid. Toxicology 2002;181-182:223-227.
- 23. Styrt B, Sugarman B, Mummaw N, White JC. Chemorepulsion of trichomonads by products of neutrophil oxidative metabolism. J Infect Dis 1991;163:176-179.
- 24. Park KJ, Ryu JS, Min DY, Lee KT. Leukocyte chemotaxis to Trichomonas vaginalis. Yonsei J Med Sci 1984;17:77-87.
- 25. Coombs GH, North MJ. An analysis of the proteinases of Trichomonas vaginalis by polyacrylamide gel electrophoresis. Parasitology 1983;86(Pt 1):1-6.
- 26. Arroyo R, Alderete JF. Trichomonas vaginalis surface proteinase activity is necessary for parasite adherence to epithelial cells. Infect Immun 1989;57:2991-2997.
- 27. Ryu JS, Kang JH, Jung SY, Shin MH, Kim JM, Park H, Min DY. Production of interleukin-8 by human neutrophils stimulated with Trichomonas vaginalis. Infect Immun 2004;72:1326-1332.
- 28. Nguyen C, Katner HP. Myeloperoxidase deficiency manifesting as pustular candida dermatitis. Clin Infect Dis 1997;24:258-260.
- 29. Aratani Y, Koyama H, Nyui SI, Suzuki K, Kura F, Maeda N. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun 1999;67:1828-1836.
- 30. Pacheco-Yépez J, Rivera-Aguilar V, Barbosa-Cabrera E, Rojas Hernández S, Jarillo-Luna . Myeloperoxidase binds to and kills Entamoeba histolytica trophozoites. Parasite Immunol 2011;33:255-264.
- 31. Hampton MB, Kettle AJ, Winterbourn CC. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect Immun 1996;64:3512-3517.
- 32. Kutter D, Devaquet P, Vanderstocken G, Paulus JM, Marchal V, Gothot A. Consequences of total and subtotal myeloperoxidase deficiency: risk or benefit? Acta Haematol 2000;104:10-15.
Fig. 1Superoxide anion production by neutrophils activated by T. vaginalis. Superoxide anion was measured on the basis of cytochrome c reduction after T. vaginalis lysate (A), membrane component (B), or excretory-secretory product (C) pretreatment. Data are mean±SD values.
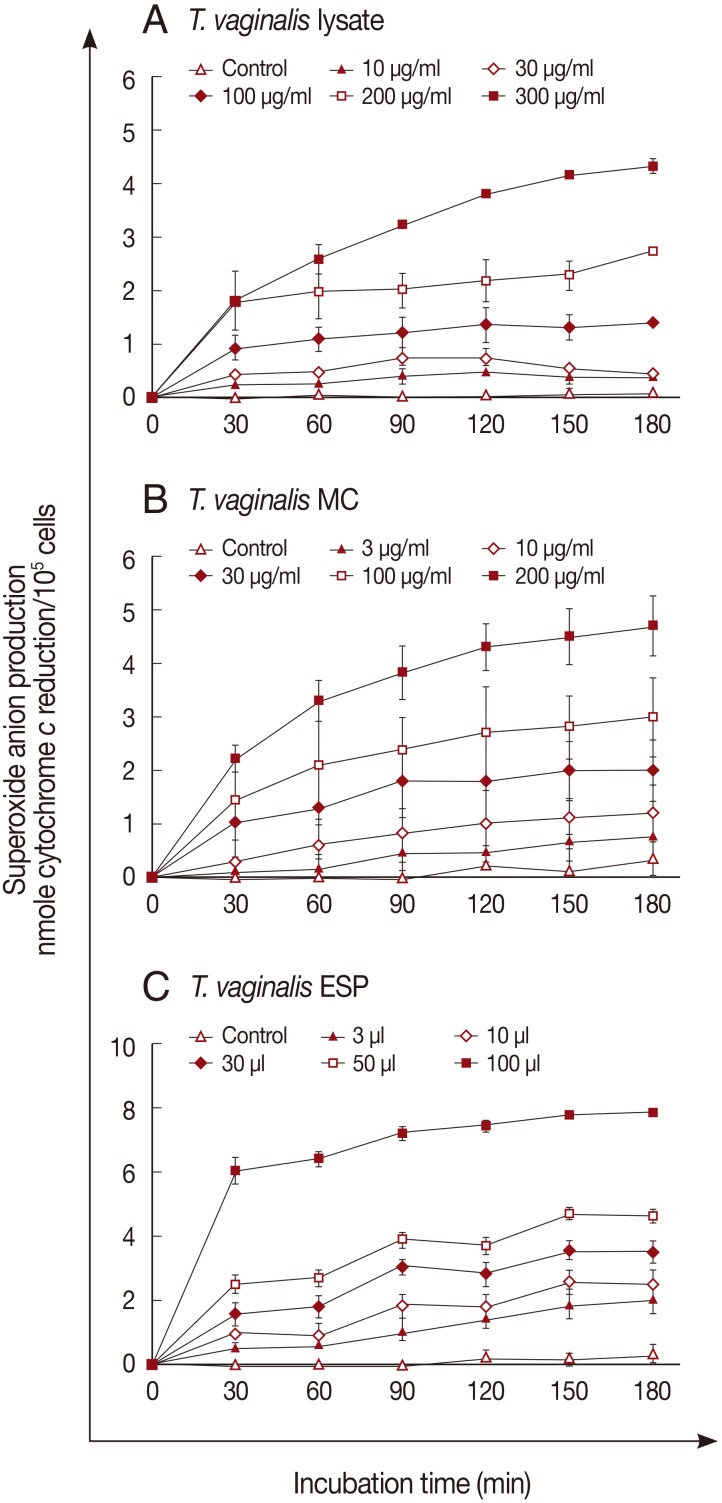
Fig. 2Effects of protease inhibitor cocktail (PIC) on superoxide anion production by neutrophils activated with T. vaginalis lysate, T. vaginalis MC, and ESP for 3 hr. Data are mean±SD values. aP<0.05.
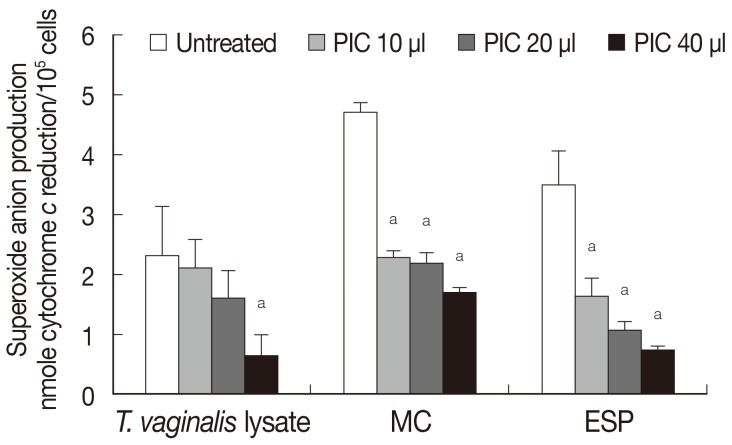
Fig. 3Effects of superoxide anion on growth of T. vaginalis. Live T. vaginalis was incubated in neutrophil-trichomonads-coculture supernatant containing superoxide anion for 3 hr, then transferred to TYM medium and incubated for 14 hr. The number of trichomonads was counted with a hemocytometer. aP<0.05.
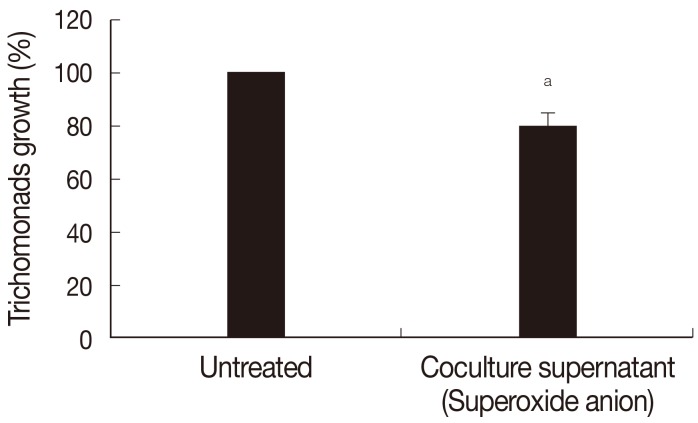
Fig. 4Myeloperoxidase production by neutrophils activated by T. vaginalis. Myeloperoxidase was determined by ELISA after coincubation of neutrophils and live T. vaginalis (N:T=10:1). Data are mean±SD values. aP<0.05.