A Rapid Diagnostic Test for Toxoplasmosis using Recombinant Antigenic N-terminal Half of SAG1 Linked with Intrinsically Unstructured Domain of GRA2 Protein
Article information
Abstract
Toxoplasma gondii is an apicomplexan parasite with a broad host range of most warm-blooded mammals including humans, of which one-thirds of the human population has been infected worldwide which can cause congenital defects, abortion, and neonatal complications. Here, we developed a rapid diagnostic test (RDT) for T. gondii infection. Antigenic N-terminal half of the major surface antigen (SAG1) was linked with intrinsically unstructured domain (IUD) of dense granule protein 2 (GRA2). The recombinant GST-GRA2-SAG1A protein was successfully expressed and purified as 51 kDa of molecular weight. Furthermore, antigenicity and solubility of the rGST-GRA2-SAG1A protein were significantly increased. The overall specificity and sensitivity of GST-GRA2-SAG1A loaded RDT (TgRDT) were estimated as 100% and 97.1% by comparing with ELISA result which uses T. gondii whole cell lysates as the antigen. The TgRDT tested with Uganda people sera for field trial and showed 31.9% of seroprevalence against T. gondii antibody. The TgRDT is proved to be a kit for rapid and easy to use with high accuracy, which would be a suitable serodiagnostic tool for toxoplasmosis.
INTRODUCTION
Toxoplasma gondii is an intracellular protozoan parasite and causes a zoonotic disease [1]. Oocysts shed by final host (cats) can be introduced into humans by eating undercooked or raw meat, or drinking water contaminated with the oocysts. Infection of pregnant women may cause severe damage such as blindness, mental retardation, encephalitis, even though fetal death to her fetus via placental transmission of T. gondii, a process known as congenital toxoplasmosis [2]. Accurate and rapid diagnosis of immune deficient individuals and prenatal diagnosis of pregnant women are important because of its capacity to induce severe clinical diseases such as encephalitis [3], ocular problems in retina with chorioretinitis [4], and congenital oxoplasmosis.
Several serological methods such as ELISA, latex agglutination test (LAT), and indirect fluorescent antibody test (IFAT) have been developed for detection of T. gondii infection, most of these methods need whole cell lysates of T. gondii as an antigen which is expensive and time-consuming to prepare. To overcome these disadvantages, detection method in the form of rapid diagnostic test (RDT) and usage of recombinant proteins as antigen have been introduced. Recently, a truncated recombinant SAG2-loaded RDT was developed and evaluated for its diagnostic properties on infected and uninfected cats [5].
A surface antigen, SAG1, is a highly abundant surface protein which is expressed on the rapidly dividing tachyzoites and mostly used as antigenic materials of the diagnostic kit to detect antibodies against T. gondii. In our previous study, rSAG1 loaded RDT showed that is rapid, easy to use, and highly accurate with 100% sensitivity and 99.2% specificity in serodiagnosis of cat toxoplasmosis [6]. For the development of SAG1 antigen with enhanced solubility and specificity as RDT antigen for serodiagnosis of human toxoplasmosis, in present study, we changed SAG1 antigen to SAG1A antigen [7] and linked with intrinsically unstructured domains (IUD) of GRA2 protein which is known to be a potential and useful diagnostic marker as they are found abundantly in both tachyzoites and bradyzoites [8] and is also able to induce strong antibody responses during acute infection [9] or acute and chronic infections [10]. In a previous report, we showed that GRA2 is a hydrophobic protein containing the N-terminal hydrophobic signal peptide and has 2 internal amphipathic alpha helices [11]. IUD have special sequences that do not have the capacity to form sufficient strong interaction, but more flexible. With these properties, IUD was tested as a useful diagnostic antigen and reported that this IUD containing antigen offers considerable advantages and diagnostic sensitivity and specificity in Echinococcus serodiagnosis [12].
In this study, we investigated antigenic properties such as the solubility of modified recombinant proteins, to be used in the development of RDT for serodiagnosis of T. gondii and developed a recombinant SAG1A (rSAG1A)-based RDT kit via GRA2 linker adaptation. Finally, we evaluated its serodiagnostic performances using serum specimens which obtained from Seoul Saint Mary's Hospital in the Republic of Korea (=Korea) and Uganda people.
MATERIALS AND METHODS
Clinical samples
A total of 67 human sera which were collected and diagnosed from Kang-Nam Saint Mary's Hospital for diagnosis of toxoplasmosis and a total of 119 human sera collected from villages near Kiboga, Uganda, conducted with approval from the Uganda Ministry of Health, and stored at -80℃ in Department of Parasitology, Inha University School of Medicine were examined by RDT kit. The results were compared with ELISA kit which has been used in Department Parasitology, Catholic Institute of Parasitic Disease, Catholic University of Korea, Seoul, Korea.
Construction of vector for GST-GRA2 linker-SAG1A plasmids
The SAG1A antigen of T. gondii obtained from nucleotide sequences (corresponding to nucleotide 145-660) of antigenic N-terminal half of the SAG1(corresponding to nucleotide 1-1011) (GenBank no. HM76940.1) by PCR using the following gene specific primers (SAG1A domain: forward primer: 5'-gttgaattcgat ccccctcttgtg cc-3' and reverse primer: 5'-gtg gaattcgactccatcttt ccc gca-3') and ligated into EcoR1 site of pGEX-4T-1 vector (GST expression vector, Amersham Pharmacia Biotech, Upssala, Sweden). For the improvement of the antigenicity and solubility, we designed the GRA2 domain (corresponding to nucleotide 94-213) of GRA2 (GenBank no. HM014012.1) as the linker which selected gene fragment based on IUD regions using bioinformatic software (IUPred). After PCR amplification using the following gene specific primers (GRA2 IUD domain: forward: 5'-cg ggatcccagggaccagtc gac-3' and reverse primer: 5'-cgggatccaacaggttcttc tgg ct-3'), BamH 1 site of the PCR product was ligated to a pGEX-4T-1/GST/SAG1A vector construct. The finally constructed vector named as GST-GRA2-SAG1A.
Preparation of rGST-GRA2-SAG1A proteins
Recombinant proteins of GST-GRA2-SAG1A and GST-SAG1A were produced in BL21 (DE3) strain of E. coli to test the antigenicity against T. gondii antibodies and purified according to the protocol previous described by Chong et al. [6]. The purified recombinant protein was separated by 12% SDS-PAGE and stained with Coomasie blue. All images were captured using the Gel Doc™ XR+ with Image Lab Software (Bio-Rad, Hercules, California, USA).
Analysis of solubility and antigenicity of recombinant protein
For the solubility analysis, E. coli cell cultures were centrifuged at 3,000 g, 4℃ for 10 min, after discard of supernatant, cell pellets resuspended in 1 ml of PBS and centrifuged at 10,000 g, 4℃ for 1 min. Pellets resuspended in 300 µl of PBS and sonicated with 6×10 sec and centrifuged at 7,000 g, 4℃ for 5 min. Finally 100 µl of 1x SDS sample buffer added into pellet or supernatant fractions. After boiling at 100℃ for 3 min, samples (soluble: supernatant/insoluble: pellets) were resolved by 12% SDS-PAGE and stained with Coomasie blue. For the antigenicity analysis, T. gondii RH whole lysates and rGST-GRA2-SAG1A and rGST-SAG1A proteins were blotted with T. gondii patient's sera. The immune complexes were detected with enhanced chemiluminescence (ECL) (GE Healthcare, Little Calfont, UK) and analyzed with Luminant Image Analysis System (LAS-3000, Fuji film, Tokyo, Japan).
Preparation and interpretation of RDT kit
Colloidal gold particles (40 nm in mean diameter) were prepared and conjugated with rGST-GRA2-SAG1A antigen according to a previously described procedure [6]. Briefly, the assay procedure was as follows: The first step was started by 10 µl sera drop onto the whole in assemble plastic cassettes and then dropping 100 µl of buffer A, i.e., 0.1% casein and 1% Tween 20 in 0.1 M Tris-HCl buffer (pH 8.0), to the sample pad. After 5 min of the buffer treatment, the results were interpreted. Control line (C) should appear in all tests as a red band. After then, if the red band is showed in the T line (under the control band), it means that anti-T. gondii antibodies contained the sample sera, so we decided as positive such as + (weak), ++ (mild), and +++ (strong antigenic reactivity).
Evaluation of sensitivity and specificity of TgRDT
To investigate the analytical sensitivity of TgRDT, we used 67 different specimens (34 positive and 33 negative) of sera from patients diagnosed with toxoplasmosis in Seoul Saint Mary's Hospital, Korea. The detection threshold level of the kit was compared with the laboratory ELISA kit [13] which has been used in Department of Parasitology, Catholic Institute of Parasitic Disease, Catholic University of Korea. The optical density (OD) of duplicated samples were measured at 490 nm with a spectrophotometer (PerkinElmer Victor3, Turku, Finland) and compensated by comparing the OD of a standard positive serum in each plate. The ELISA index of 0.25 were determined as positive cut-off values. Comparison between groups was analyzed by the Student's t-test.
RESULTS
Construction of GST-GRA2 linker-SAG1A in pGEX-4T-1 vector
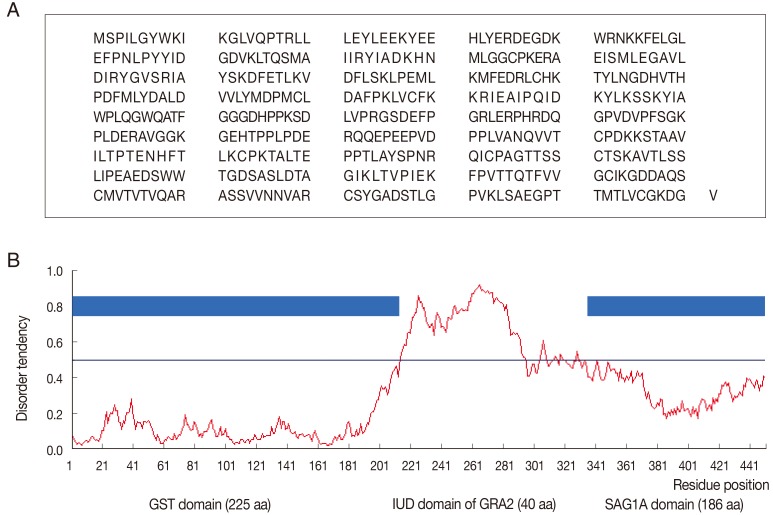
The PCR products of SAG1A fragment (558 bp) and GRA2 fragment (120 bp) as the linker of IUD were successfully ligated into the pGEX-4T-1 plasmid. The constructed GST-GRA2-SAG1A retained the open reading frame encoding 451 amino acid residues (Fig. 1A), and the globular domain contained GST domain (1-225), GRA2 linker domain (226-265), and SAG1A domain (269-451). The predicted structure of GST-GRA2-SAG1A showed that the putative IUD region had a high prediction score (score of over 0.5) (Fig. 1B).
Characterizations of rGST-GRA2-SAG1A proteins
The rGST-GRA2-SAG1A protein was successfully expressed in E. coli BL21(DE3) and purified as a 51 kDa of molecular weight compared with GST-SAG1A protein (45 kDa) (Fig. 2A) and showed an increased solubility compare with GST-SAG1A protein (Fig. 2B-1/B-2). Furthermore, rGST-GRA2-SAG1A protein represented more enhanced antigenicity when reacted with toxoplasmosis diagnosed patient's sera (Fig. 2C-2/2C-3).
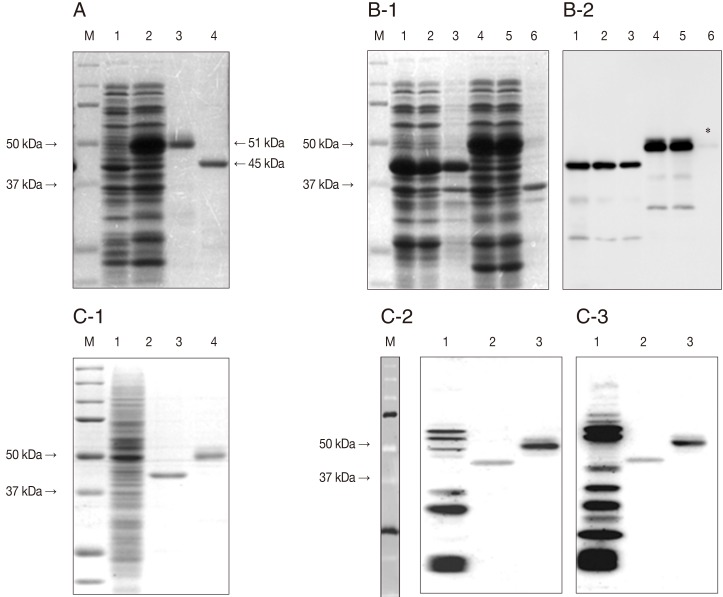
Expression, purification, solubility, and antigenicity of recombinant GST-GRA2-SAG1A proteins. (A) Expression and purification of rGST-GRA2-SAG1A proteins in E. coli BL21(DE3). Cell lysates were separated on a 12% SDS-PAGE gel. The protein was stained with Coomassie blue. Lane M, dual protein marker; lane 1, sonicates of E. coli/GST/GRA2-SAG1A before induction; lane 2, sonicates of E. coli/GST/GRA2-SAG1A after induction with 1 mM IPTG; lane 3, purified rGST-GRA2-SAG1A protein; lane 4, purified rGST-SAG1A protein. (B) Solubility of rGST-GRA2-SAG1A proteins in E. coli. Lane M, dual protein marker; lane 1, whole cell lysates of E. coli/-GST-SAG1A after induction; lane 2, soluble part of E. coli/GST-SAG1A after induction; lane 3, insoluble part of E. coli GST-SAG1A after induction; lane 4, whole cell lysates of E. coli/GST-GRA2-SAG1A after induction; lane 5, soluble part of E. coli/GST-GRA2-SAG1A after induction; lane 6, insoluble part of E. coli/GST-GRA2-SAG1A after induction. Coomassie blue stain (B-1) and western blot with anti-GST antibody (B-2) (asterisk indicates molecular weight of insoluble part of rGST-GRA2-SAG1A). (C) Antigenicity of rGST-GRA2-SAG1A proteins. Proteins were stained with Coomassie blue. Lane M, dual protein marker; lane 1, cell lysates of T. gondii RH strain; lane 2, purified rGST-SAG1A protein; lane 3, purified rGST-GRA2-SAG1A protein (C-1). Western blot with toxoplasmosis patients sera (C-2/C-3).
Analytical specificity and sensitivity of TgRDT with rGST-GRA2-SAG1A protein
To evaluate the usage of rGST-GRA2-SAG1A protein as a diagnostic antigen, the recombinant protein was loaded into the RDT kit and tested with 67 human sera. Interpretation was according to the protocol which was described in Materials and Methods (Fig. 3A) and compared with ELISA results (Fig. 3B). The newly developed TgRDT showed concordance with 33 sera in 34 ELISA-positive sera but did not react with 33 negative sera. Therefore, the overall specificity and sensitivity of the TgRDT were 100% and 97.1%, respectively (Table 1). Two samples (grey-colored circle) which showed high OD values (>1.0) in ELISA were reacted with TgRDT as weak or mild. It was suggested that these sera may be chronic infected sera, so there were many antibodies against various antigens such as SAGs, GRAs, and MICs of T. gondii detectable with ELISA because ELISA uses T. gondii whole cell lysate as antigen but TgRDT uses only SAG1A antigen. Another 4 samples (gradated circles) which showed low OD values (0.3-0.7) in ELISA showed strong reactivity in TgRDT (Fig. 4). This result means that these sera may have been obtained from early or acute patients, so there were many SAG1 antibodies compared with any other antigens of T. gondii.

Comparison of ELISA and TgRDT of Korean sample. (A) Interpretation of TgRDT. Normal or negative: only 1 red upper band. Positive: + means weak, ++ mild, +++ strong antigen reactivity indicated as band. (B) Comparison of ELISA and TgRDT. Serum ELISA index of 0.25 was determined as the cut-off point.
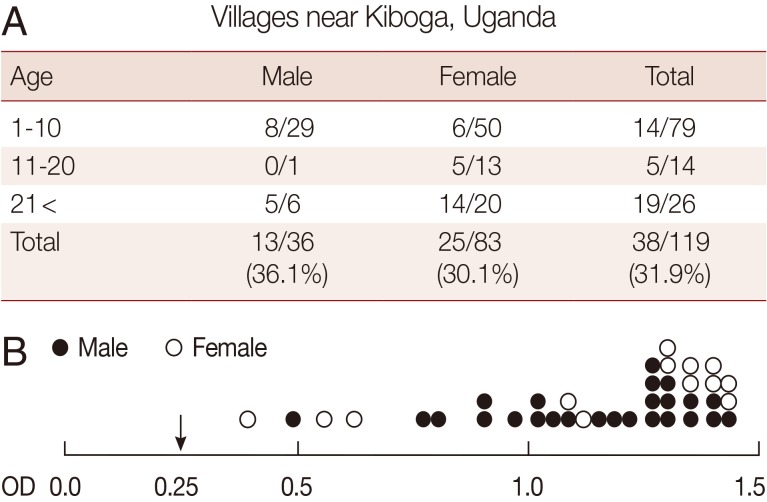
Seroprevalence of T. gondii in Uganda people sera
Total 119 of Uganda people sera were tested for seroprevalence of T. gondii. As a result, the seroprevalence against T. gondii antibody was 31.9% (Fig. 4A). There was no significant difference in TgAb-positive rates between males (36.1%) and females (30.1%). The TgRDT positive sera showed highly OD values (<0.4) in ELISA (Fig. 4B).
DISCUSSION
Many kinds of recombinant proteins of T. gondii were studied as potential candidates for diagnostic antigens to detect its antibodies in cats and also in humans. There were many trials for more effective, sensitive, and specific antigens. Recently, a fly-casting model of IUProtein recognition [14], which illustrates a relatively unstructured disordered protein molecule, was introduced, and was revealed to have a greater capture radius for a specific binding site through more increased flexibility [15,16]. Well-established IUD domain's recognition model was reported in calmodulin plasticity, which revealed how the central helix unwinds in order to position the 2 domains optimally in the recognition of different target molecules [17]. Flexible linker's roles are well explained in protein-protein interaction. It has become clear that unfolded flexible regions of proteins do have a major function [18]. In the present study, the enhanced antigenicity of rGST-GRA2-SAG1A protein was probably linked with the IUD region of GRA2 protein and increased solubility may also have been induced by linkage with the IUD region of GRA2 protein which as known as a secreted protein.
Various recombinant proteins such as rhoptry proteins (rROP1 and rROP2), dense granule proteins (rGRA1, rGRA2, rGRA4, rGRA6, rGRA7, and rGRA8), matrix proteins (rMAG1), microneme proteins (rMIC2, rMIC3, rMIC4, and rMIC5), and surface antigens (rSAG1 and rSAG2) were evaluated for their potentials as diagnostic antigens for diagnosis of toxoplasmosis in humans [19]. Among them, SAG1 is one of the most suitable proteins because of its structural abundance in T. gondii and better antigenic properties [20]. Except for Wu et al. study [21] which showed IgG-ELISA assay of rSAG1 (61-290 aa) with 93.9% sensitivity and 100% specificity in human sera, many studies did not show excellent results. In the case of Buffolano et al. [22], they reported that rSAG1 showed 65.7% sensitivity and 95.8% specificity in human infant sera. Pferepper et al. [23] reported that among tested recombinant antigens (rROP1, rMAG1, rSAG1, rGRA7, and rGRA8), SAG1 showed 29.4% in acute cases and 91.5% in chronic cases in IgG positive rate. In this study, we demonstrated that rGST-GRA2-SAG1A protein strongly reacted with T. gondii antibodies in human sera and the recombinant protein could replace somatic antigens of T. gondii in the diagnosis. The rGST-GRA2-SAG1-loaded TgRDT showed very good analytical sensitivity in our experiments. The structurally modified recombinant protein produced excellent results of clinical assessment of TgRDT (100% specificity and 97.1% sensitivity). With these results, we suggest that our newly developed TgRDT is highly useful as a serodiagnostic tool in acute or chronic infections of T. gondii.
Many African countries suffer from HIV/AIDs and exhibit a high seroprevalence of T. gondii. In recent serodiagnostic study of HIV-patients from Uganda, high T. gondii seroprevalence of 54% was reported [24]. In our study, the seroprevalence was 31.9% among random group sera and it was presented that these sera were from chronic or reinfected people because these sera showed high ELISA OD values and strongly reacted with TgRDT. There was no significant difference in the TgAb-positive rates between men and women or age distribution. It is generally known that no gender difference is recognized in T. gondii prevalence [25]. This newly developed TgRDT kit is the first TgRDT kit to diagnosis T. gondii-specific antibodies in humans. It is very simple to use, rapid to assay, and very sensitive and highly specific. Therefore, it would serve as a choice of method for point-of-care diagnosis and large scale surveys of T. gondii infection among people under clinical or field conditions in the worldwide endemic areas. Finally, this is probably the first and noble trial in the development of RDT kit with IUD linked recombinant antigen for serodiagnosis of human toxoplasmosis.
ACKNOWLEDGMENT
This research was partially supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2012R1A1A 2002612).
Notes
We have no conflict of interest related with this work.