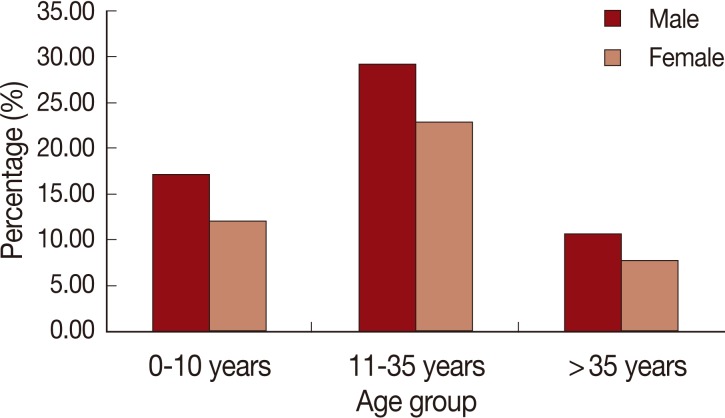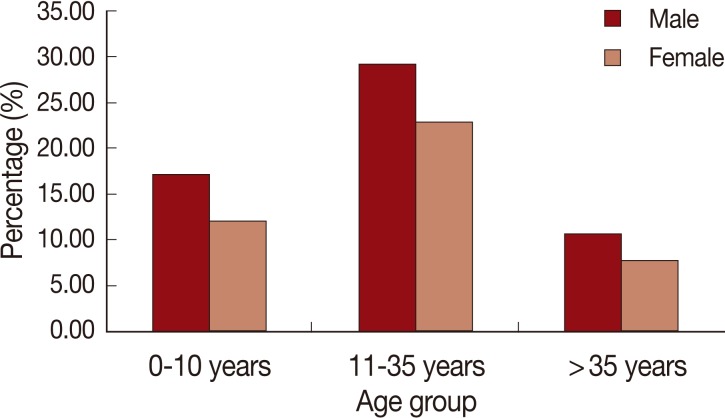Abstract
Cutaneous leishmaniasis (CL) is an endemic parasitic infection in the Mediterranean region, including Libya and its Al-jabal Al-gharbi province. We aimed at studying the occupational relevance as well as other epidemiological aspects of CL. We investigated 140 CL cases who attended at Gharyan outpatient polyclinic during a period of 6 months in 2009. CL infection was clinically diagnosed and confirmed by demonstration of Leishmania parasites on smears from lesions. Our findings showed that males were more affected than females (P=0.04), and people above 10-years were more affected than younger ones (P=0.0001). A significant percent of CL cases belonged to Al-Kawasem subprovince (P=0.0001). Farm-related activities were the most frequent occupations among CL cases (P=0.04). In addition to farm workers, housewives and students are at risk groups since they are engaged at farm activities. Moreover, those who have occupations that require staying outdoors for a part of night, e.g., policemen, are also at risk. Compared to children, adult CL patients had multiple lesions (P=0.001) that were more prevalent in their upper and lower extremities than the face (P=0.0001). We conclude that CL is a major health problem in Al-jabal Al-gharbi province of Libya. The presence of rodents and sandflies makes it a suitable environment for Leishmania to spread in an endemic epidemiological pattern. Being engaged in farming activities or outdoor occupations increases the risk of infection. Various clinical patterns of CL suggest the presence of more than 1 species of Leishmania at Al-jabal Al-gharbi province. We propose that the 2 species responsible for CL in this area are L. major and L. tropica. Further investigations to identify the leishmanial species responsible for CL at Al-jabal Al-gharbi together with adoption of preventive and control programs are needed.
-
Key words: Leishmania major, Leishmania tropica, cutaneous leishmaniasis, farm-related occupation, Gharyan, Libya
INTRODUCTION
Cutaneous leishmaniasis (CL), a protozoan skin infection, is a major public health problem that affects humans in different areas of the world. CL was considered mainly an occupational disease, infecting people involved in outdoor activities in farms, forests such as wood and mineral exploitation or in building of roads in forests or of hydroelectric dams [
1].
Leishmaniasis is a group of diseases caused by parasitic protozoa of the genus
Leishmania that are transmitted to humans by the bite of a blood-feeding insect female sandfly (
Phlebotomus in the old world leishmaniasis and
Lutzomyia in the new world leishmaniasis). Rodents serve as reservoir hosts for
Leishmania parasite [
2].
Five species are medically important causes of old world leishmaniasis;
Leishmania major,
Leishmania tropica and
Leishmania aethiopica, predominantly causing CL, and
Leishmania donovani and
Leishmania infantum, predominantly causing visceral leishmaniasis (VL). These species differ from each other clinically and epidemiologically. Humans are important hosts for
L. tropica and
L. donovani, while the great gerbil, rock hyrax, and domestic dogs are mammalian hosts for
L. major,
L. aethiopica, and
L. infantum, respectively [
3].
New world leishmaniasis is caused by 2 major groups;
Leishmania mexicana and
Leishmania braziliensis complexes.
L. mexicana complex includes 5 species of which
L. mexicana and
Leishmania pifanoi are the most important. The remaining 3 species are
Leishmania amazonensis,
Leishmania garnhami, and
Leishmania venezuelensis. Although all members of
L. mexicana complex cause CL, each of them shows a unique clinical presentation. Species of
L. braziliensis complex include
Leishmania peruviana,
Leishmania lainsoni,
Leishmania colombiensis,
Leishmania guyanensis, and
Leishmania panamensis. The first 3 species of
L. braziliensis complex cause mucocutaneous leishmaniasis while the last 2 species can cause both cutaneous and mucocutaneous leishmaniasis. VL in the new world is caused by other leishmanial species called
Leishmania chagasi [
3].
Based on the causative agent and clinical presentation, it is clearly known that leishmaniasis is classified into 3 types; cutaneous, mucocutaneous, and visceral leishmaniasis. The most common clinical presentation of CL is localized ulcer or nodule. CL is endemic in over 80 countries all over the world, where approximately 300,000 cases of CL are reported, annually [
3].
In the Eastern Mediterranean Region (EMR) of the World Health Organization (WHO), leishmaniasis represents a major public health problem. Cutaneous and visceral leishmaniasis are mainly seen in 14 of 22 countries of the region. In several of these countries outbreaks have an apparent tendency to occur at around 10-year intervals. Foci of CL, caused by
L. major, occur in Afghanistan, Egypt, Iran, Iraq, Jordan, Libya, Morocco, Palestine, Pakistan, Saudi Arabia, Sudan, Syria, Tunisia, and Yemen [
4]. In 2008, some 100,000 new cases of CL were reported by the EMR-WHO. Many researchers have studied leishmaniasis in the endemic northern African countries, e.g., Morocco, Algeria, Tunisia, Egypt, and Libya [
5-
12]. One of the established endemic leishmaniasis Libyan provinces is Al-jabal Al-gharbi province, where CL comprises a major parasitic health problem [
7,
10-
12].
The aim of the present study is to investigate the clinical patterns and detailed occupational and geographical distribution with some other epidemiological aspects of CL among cases attending Gharyan outpatient polyclinic, Gharyan, Al-jabal Al-gharbi, Libya.
MATERIALS AND METHODS
Surveyed area and patients
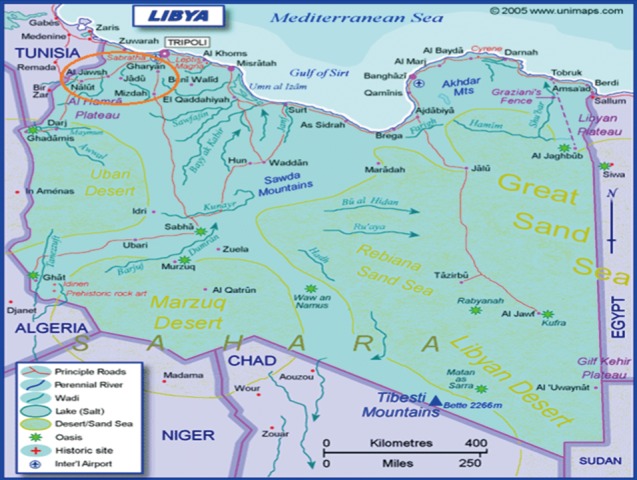
This study was performed at Al-jabal Al-gharbi province (formerly known as Jabal Nafusah;
Fig. 1), which is located south west of the Libyan capital; Tripoli. It is divided into 35 subprovinces with a total population of 302,000 individuals. Gharyan subprovince is the administrative city of the Al-jabal Al-gharbi province (
Fig. 1).
Patients with CL attending at Gharyan outpatient polyclinic, Gharyan, Al-jabal Al-gharbi, Libya, during a 6-month period from 1 December, 2008 till 31 May, 2009 were included in this study. Gharyan outpatient polyclinic is the only leishmaniasis clinic in Al-jabal Al-gharbi province, to which all cases of CL are referred by other clinics to seek medical diagnosis, treatment, and consultation. At Gharyan polyclinic, those CL cases were clinically diagnosed and confirmed by laboratory investigations. A prepared questionnaire has been filled out for each case. The questionnaire included personal, clinical, occupational, and epidemiological data. Skin smears for microscopic testing and photographs have been taken for the CL lesions. Skin smears were taken from the active part of lesions, left to be air-dried, stained by Giemsa, and screened for the presence of amastigote forms of Leishmania species under a light microscope.
Clinical presentation
Clinical presentation of the reported patients confirmed that it was CL lesions; the absence of the involvement of the mucous membrane ruled out the possibility of being mucocutaneous leishmaniasis and the absence of lymphadenopathy and hepatosplenomegaly excluded the diagnosis of visceral leishmaniasis. Three clinical forms of the CL lesions were observed in our study; papulo-ulcerated, nodulo-ulcerative, and indurated ulcerated scaly plaque form.
Light microscopic diagnosis
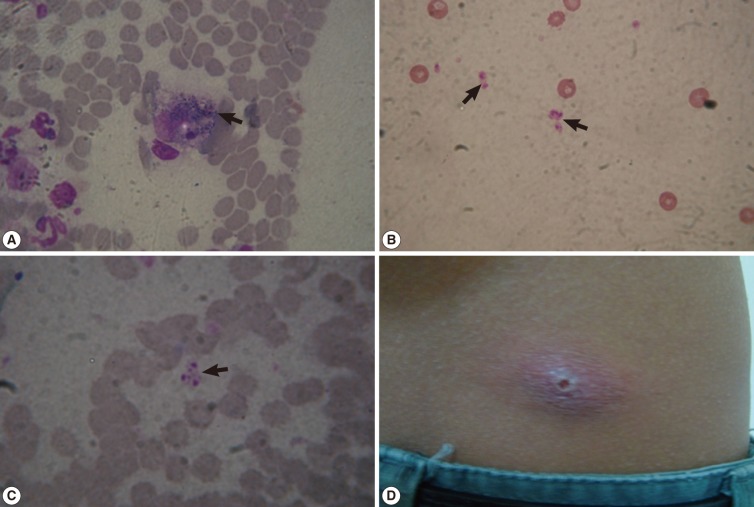
The Giemsa stained smears confirmed our preliminary clinical diagnosis as they revealed the presence of leishmanial parasites. Leishmanial parasites appear as amastigotes that multiply within macrophages (
Fig. 2A). Amastigotes are ovoid or round and 1.5-3.0 µm in diameter. They have thin cell membrane, a relatively large nucleus, and a rod-shaped kinetoplast (
Fig. 2B, C).
CL patients were treated with one of the following therapeutic strategies; local liquid nitrogen cryotherapy alone (which was applied once a week for 1-8 times according to the patient's response), a combination of systemic antibiotic and local liquid nitrogen cryotherapy (the antibiotic was prescribed for 1 week before the start of cryotherapy to treat secondary bacterial infections) or administration of antimony compounds alone (pentostam which was administered as sodium stibogluconate intramuscularly or intralesionaly at a dose of 20 mg/kg body weight daily for 20 days and may be repeated with 10-day interval). A 46.4% of the reported CL cases were treated with only local liquid nitrogen cryotherapy applied at the peripheries of the lesions once a week for 1-8 times according to the individual's response and the number of lesions, 36.4% of the reported CL cases were treated with a combination of systemic antibiotics (which was used to be prescribed 1 week before the application of cryotherapy) and local liquid nitrogen cryotherapy, while the remaining CL cases (17.1%) were treated with systemic treatment with antimony compounds (pentostam).
Ethical approval
An informed consent was obtained from each adult patient or the responsible person for the children cases; and the study was approved by the ethical reviewing board of Gharyan hospital and Faculty of Medicine, Al-jabal Al-gharbi University, Libya.
Statistical analysis
The obtained data were statistically analyzed using the statistical package for social sciences program, version 16 (SPSS-16). Frequency distribution, descriptive statistics, chi-square and T-test were performed whenever needed.
RESULTS
Geographic distribution of cases and demographic findings
During the 6-month period of the study that started from the beginning of December 2008 till the end of May 2009, 140 patients with CL attended at Gharyan outpatient polyclinic. Those cases belonged mainly to certain subprovinces, e.g., Al-Kawasem, Banikhalifa, Taghasat, Asabaa, Alorban, Rabtah, Mizdah, Kekla, Zantan, etc. Among the 140 CL cases, there were 67 children (47.9%) and 73 adults (52.1%). Males were significantly more affected than females, where there were 80 males (57.1%) and 60 females (42.9%) among the reported 140 CL cases (z=1.71, P=0.04). CL was more prevalent in patients aged above 10 years (n=99; 70.7%) than patients whose ages were 10 years or younger (n=41; 29.3%) (z=5.39, P=0.0001).
Among the 140 CL cases, patients from Al-Kawasem subprovince (n=39; 27.9%) were significantly overrepresented than other subprovinces (z=4.69, P=0.0001). Being engaged in farm activities is considered as a risk factor for exposure to CL infection. Farm-related occupations were the most frequent job "26.0%" among CL reported adult cases (z=1.74, P=0.04). In addition to farm workers, most students and housewives are at risk groups since they are engaged in farm activities and those who have occupations that require staying outdoors for a part of the night, e.g., policemen. Among children, there was no difference regarding the occurrence of single or multiple lesions (z=0.122, P=0.45), while among adults multiple lesions (n=48) were significantly prevalent than single (n=25) lesions (z=3.90, P=0.035). Distribution of CL lesions on body parts was significantly different between children and adults. In adults, upper and lower extremities were significantly more affected than other body parts while in children the face was the most affected site (χ2=38.3, P=0.0001).
Clinical findings
Regarding the clinical findings in this study, the majority of CL lesions were seen on the exposed parts of the body such as the face and upper and lower extremities. The size of CL lesions ranged from 0.5 cm to 20 cm with a mean of 3.7±3.5 cm. The delay before diagnosis and initial presentation of CL lesions ranged from 0.5 month to 8 months with a mean of 2.3±1.4 months. Three clinical forms of CL were observed; single or multiple papulo-ulcerated (35.7%), nodulo-ulcerative (59.3%), and indurated ulcerated scaly plaque form (5%) (
Figs. 2D,
3,
4,
5D). Typically, CL starts as a small, erythematous papule at the site where promastigotes are inoculated by the bite of a sandfly. The lesions progress from papules to nodules to non-ulcerated dry plaques or ulcers (with a raised indurated border and central depression) that are usually painless unless secondarily infected (
Figs. 4C,
5D). These CL lesions may be single or multiple primary lesions and/or satellite lesions (
Fig. 5C). The least observed clinical presentation was the indurated ulcerated scaly plaques which are characterized by the necrotic tissue and plaques formation (
Fig. 5). Among the patients, 121 (86.4%) were not inflamed at their initial presentation at Gharyan outpatient polyclinic while the rest of the patients (13.6%) presented with inflamed lesions due to secondary bacterial infections. Among the non-inflamed CL lesions, 22.9% were inflamed during their treatment with local application of liquid nitrogen that required specific treatment with antibiotics.
Treatment of the single lesions was mostly recovered by application of cryotherapy, while CL cases with multiple lesions might not respond to cryotherapy alone, and it was not uncommonly required systemic treatment with antimony compounds. However, all of the 3 treatment protocols were followed by complete healing of the CL lesions while leaving disfiguring scars.
DISCUSSION
CL is considered an endemic disease in Libya. One of the Libyan endemic provinces is Al-jabal Al-gharbi [
7,
10-
12]. Our present study aimed to compare the percentage, clinical patterns, detailed geographical distribution, and other epidemiological aspects of CL among the reported cases from various endemic subprovinces at Al-jabal Al-gharbi, Libya. Total 140 CL cases were registered at Gharyan outpatient polyclinic during the study period from 1 December, 2008 till 31 May, 2009.
Our results showed that the percentage of children and adult cases were approximately equal. Males were significantly more affected than females, where there were 80 males (57.1%) and 60 females (42.9%) among the reported 140 cases (z=1.71,
P=0.04) (
Table 1). However, in a study that was conducted in southeastern France [
13], males and females were equally affected with CL.Other studies in North-central province of Sri Lanka [
14] and Al-Badarna, Libya [
5] have shown similar findings to our results, where males were significantly more affected than females. This sex difference can be attributed to the engagement of Libyan males in outdoor activities and night duties that exposes them to sandfly bites more than females.
When studying the effect of age, we found that the most affected age group was individuals whose ages ranged between 11 and 35 years of both sexes (
Fig. 6). Our results nearly coincided with the result of other reports from southern Sri Lanka [
15], Pakistan [
16], and Turkey [
17] where the most affected age groups were 10-19, 10-14, and 9-14 years, respectively. However, a previous survey in Al-Badarna, Libya reported that the most affected age group was 1-10 years [
10]. Therefore, on analysis of data we classified CL patients into 2 main age groups; the first included children up to 10 years of age and the second group included patients whose ages were above 10 years. CL was more prevalent in patients above 10 years (n=99; 70.7%) than patients whose ages were 10 years or younger (n=41; 29.3%) (z=5.39,
P=0.0001). This can also be explained by the risk of exposure to sandfly bites when engaged in outdoor activities performed by people over the age of 10 years than younger individuals (
Table 1).
Among the 140 CL cases, patients from Al-Kawasem subprovince (n=39; 27.9%) were significantly overrepresented than those from other subprovinces (z=4.69,
P=0.0001). Subprovinces such as Banikhalifa and Rabtah followed Al-Kawasem regarding the frequency of the reported CL cases (
Table 2). It was evident that the majority of the reported CL cases belonged to rural areas of Al-jabal Al-gharbi subprovinces, which confirms that rural areas are more exposed to leishmanial infections because of the presence of suitable environmental conditions for spread of reservoir hosts and vectors. Another reason could be the nature of rural areas where people are engaged in farming activities, working outdoors, and staying there for long till night.
CL is considered as an occupational infection. Soldiers, miners, policemen, farm, and forest workers are at high risk for CL infection [
18]. Our results showed that occupational exposure is an important determinant of infection with CL, where farm workers (26.0%) were the most affected occupational category of the affected adults (z=2.89,
P=0.001). Most of the residents of Al-jabal Al-gharbi are engaged in farm activities; growing olive, almond, and fig trees. Traditionally, the farm workers get help from the rest of their family members, including their wives, adult members, or even young children aging 10 years or more. Therefore, besides the farm workers (26.0%), there were 2 affected groups with CL; students and housewives, comprising 24.7% and 23.3%, respectively (
Table 3). Other occupations such as policemen were also considered to have a risk of exposure to CL due to the nature of their jobs that obliges them to stay outdoors till night time and exposes them to insect bites and CL infection. Therefore, farming activities and policemen occupations were significantly overrepresented among the CL cases in this study (z=5.78,
P=0.0001).
In the same respect,
Table 4 shows that schoolchildren were 73.1%, while preschool children were 26.9% (z=4.3,
P=0.0001). This significant difference refers to offering assistance by school children to their families in farm activities where there is a higher risk for encountering CL infection than preschool children. These results, however, are not compatible with the results of a study conducted in Iran where preschool children were more affected [
19], but our findings can be attributed to outdoor activities and duties of schoolchildren than preschool children.
Studying the number of lesions in CL cases involved in this study showed that the prevalence of multiple lesions is significantly higher among adult patients than single lesions (χ
2=3.90,
P=0.035). However, there was no difference between children and adults or males and females regarding the occurrence of single or multiple lesions (
Table 5).
Table 6 revealed that there were 3 main types of lesions; nodulo-ulcerative, papulo-ulcerated, and indurated ulcerated scaly plaques. In all ages and both sexes, the number of nodulo-ulcerative and papulo-ulcerated lesions was significantly higher than the indurated ulcerated-scaly plaques (
P<0.01).
Table 7 shows that wet CL lesions were significantly prevalent among adults and children and both sexes when compared with the dry CL lesions (
P<0.001).
Although our study had shown that the exposed areas of the skin were commonly affected as clearly shown in
Table 8, the distribution patterns of CL lesions in various parts of the body showed a significant difference between children and adults. In children, the face was the most frequently affected part, while in adults the upper and lower extremities were the most affected body parts (χ
2=38.3,
P=0.0001). Generally, the number and percentage of lesions in each part of the body were more in males than in females (
Table 8). CL patients confirmed the presence of rodents (126; 90.0%) and sandflies (131; 93.6%) in their localities and 40% of them mentioned the presence of similar CL lesions in other members of their families (
Table 9).
The geographical nature of Al-jabal Al-gharbi province makes it a suitable place for breeding and spreading of many kinds of reservoir hosts for
Leishmania, especially dogs and rodents as well as the transmission vector, sandfly. Moreover, the wide range of clinical presentation of CL lesions that were presented and treated at Gharyan polyclinic suggests that there are various
Leishmania species causing the reported lesions. Similar findings were observed in recent studies in Suriname [
20] and Pakistan [
21]. In Suriname, it was believed long that CL is caused by
L. guyanensis only, but recently it was found that other leishmanial species such as
L. lainsoni and
L. amazonensis are also responsible for the disease in this area [
20]. While, in Pakistan, where CL is an endemic infection, recently investigations of an outbreak of CL in Sindh province proved that
L. tropica and
L. major were traced as causes of the disease [
21]. Therefore, further studies are required to identify the various species of
Leishmania parasites responsible for CL in Al-jabal Al-gharbi, Libya.
We conclude that CL is a major health problem in Al-jabal Al-gharbi province of Libya. The presence of rodents and sandflies makes it a suitable environment for
Leishmania to spread in an endemic epidemiological pattern. Being engaged in farming activities or occupations that requires staying outdoors for long periods and till night times increases the risk of encountering
Leishmania infection. Furthermore, the observed various clinical patterns of CL that were presented with diverse types of single and multiple lesions and different geographical distributions suggest that there may be more than 1 species of
Leishmania responsible for CL among the Libyan population living at Al-jabal Al-gharbi province. Ashford and his colleagues [
7] guessed that
L. major is the most probable cause of the disease in this area according to their ecological studies. We propose that there are 2 species in this area (
L. major and
L. tropica) responsible for the wet and dry CL, respectively.
Further investigations to identify the Leishmania species responsible for CL at Al-jabal Al-gharbi are needed. Adoption of special programs to control and prevent CL must start, including health education of the public, control measures for rodents and dogs, eradication of sandflies as well as application of indoor and outdoor self-protection measures against sandfly bites are necessary especially for the population at risk.
References
- 1. Lainson R. Ecological interactions in the transmission of the leishmaniasis. Philos Trans R Soc Lond B Biol Sci 1988;321:389-404.
- 2. Coller LH, Sussman M, Balows A, Mahy BWJ. Topley and Wilson's Microbiology and Microbial Infections. Parasitology. 1998, Vol 5:9th ed. London. Arnold.
- 3. Markell EK, John DT, Krotoski WA. Markell and Voge's medical parasitology. 1999, 8th ed. Philadelphia, USA. WB Saunders.
- 4. Postigo JA. Leishmaniasis in the World Health Organization Eastern Mediterranean Region. Int J Antimicrob Agents 2010;36:S62-S65.
- 5. Kimutai A, Ngure PK, Tonui WK, Gicheru MM, Nyamwamu LB. Leishmaniasis in Northern and Western Africa: a review. Afr J Infect Dis 2009;3:14-25.
- 6. Rhajaoui M, Sebti F, Fellah H, Alam MZ, Nasereddin A, Abbasi I, Schönian G. Identification of the causative agent of cutaneous leishmaniasis in Chichaoua province, Morocco. Parasite 2012;19:81-84.
- 7. Ashford RW, Chance ML, Ebert F, Schnur LF, Bushwereb AK, Drebi SM. Cutaneous leishmaniasis in the Libyan Arab Republic: distribution of the disease and identity of the parasite. Ann Trop Med Parasitol 1976;70:401-409.
- 8. Mehabresh MI, El-Mauhoub MM. Visceral leishmaniasis in Libya--review of 21 cases. Ann Trop Paediatr 1992;12:159-163.
- 9. Mehabresh MI. Visceral leishmaniasis: new foci of infection in Libya. J Trop Med Hyg 1994;97:282-285.
- 10. el-Buni A, Ben-Darif A. Cutaneous leishmaniasis in Libya: epidemiological survey in AL-Badarna. Parassitologia 1996;38:579-580.
- 11. el-Buni AA, Ben-Darif AL, Taleb I, Refai A. Cutaneous Leishmaniasis in Al-Badarna: a prospective study among school children. Arch Inst Pasteur Tunis 1998;75:19-20.
- 12. el-Buni AA, Jabeal I, Ben-Darif AT. Cutaneous leishmaniasis in the Libyan Arab Jamahiriya: a study of the Yafran area. East Mediterr Health J 2000;6:884-887.
- 13. del Giudice P, Marty P, Lacour JP, Perrin C, Pratlong F, Haas H, Dellamonica P, Le Fichoux Y. Cutaneous leishmaniasis due to Leishmania infantum. Case reports and literature review. Arch Dermatol 1998;134:193-198.
- 14. Siriwardena HV, Udagedara CU, Karunaweera ND. Clinical features, risk factors and efficacy of cryotherapy in cutaneous leishmaniasis in Sir Lanka. Ceylon Med J 2003;48:10-12.
- 15. Rajapaksa US, Ihalamulla RL, Udagedra C, Karunaweera ND. Cutaneous leishmaniasis in southern Sir Lanka. Trans R Soc Trop Med Hyg 2007;101:799-803.
- 16. Anwar M, Hussain MA, Ur-Rehman H, Khan I, Sheikh RA. Epidemic of cutaneous leishmaniasis: 109 cases in a population of 500. East Mediterr Health J 2007;13:1212-1215.
- 17. Sucakli MB, Saka G. Epidemioloy of cutaneous leishmaniasis in Diyarbakir (2002-2006). Turkiye Parazitol Derg 2007;31:165-169.
- 18. Kweku MA, Odoom S, Puplampu N, Desewu K, Nuako GK, Gyan B, Raczniak G, Kronmann KC, Koram K, Botero S, Boakye D, Akuffo H. An outbreak of suspected cutaneous leishmaniasis in Ghana: lessons learnt and preparation for future outbreaks. Glob Health Action 2011;4.
- 19. Yaghoobi-Ershadi MR, Akhavan AA, Zahraei-Ramazani AV, Abai MR, Ebrahimi B, Vafaei-Nezhad R, Hanafi-Bojd AA, Jafari R. Epidemiological study in a new focus of cutaneous leishmaniasis in the Islamic Republic of Iran. East Mediterr Health J 2003;9:816-826.
- 20. van der Meide WF, Jensema AJ, Akrum RA, Sabajo LO, Lai A Fat RF, Lambregts L, Schallig HD, van der Paardt M, Faber WR. Epidemiology of cutaneous leishmaniasis in Suriname: a study performed in 2006. Am J Trop Med Hyg 2008;79:192-197.
- 21. Shoaib S, Tauheed S, Hafeez A. Cutaneous leishmaniasis: an emerging childhood infection. J Ayub Med Coll Abbottabad 2007;19:40-41.
Fig. 1Libyan map showing the location of Aljabal Algharbi province and some of its subprovinces (inside the red circle).
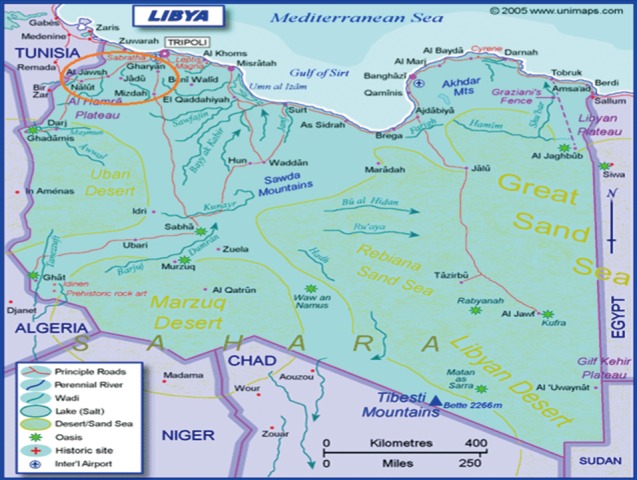
Fig. 2(A) Giemsa-stained skin smear showing amastigote forms (arrow) of Leishmania species inside a macrophage (×1,000), (B, C) Giemsa-stained skin smears showing scattered ovoid or round amastigotes (arrows) measuring 1.5-3.0 µm in diameter with a thin cell membrane, a relatively large nucleus, and a rod-shaped kinetoplast (×1,000). (D) A single nodulo-ulcerated cutaneous leishmaniasis lesion on the back of an adult patient.
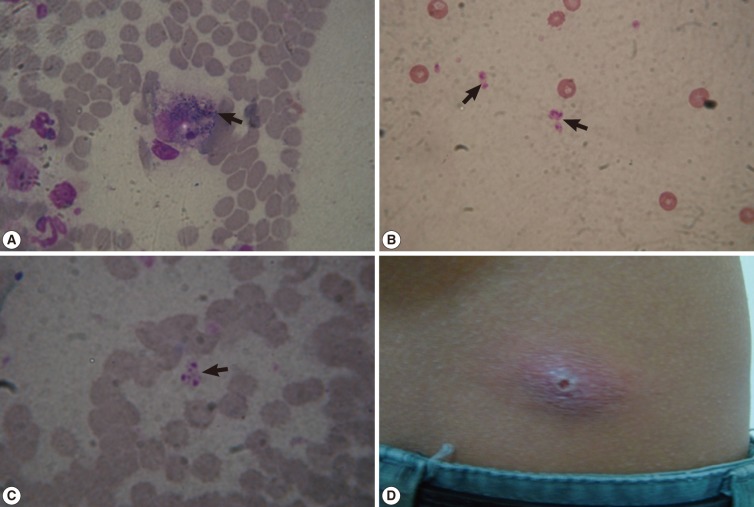
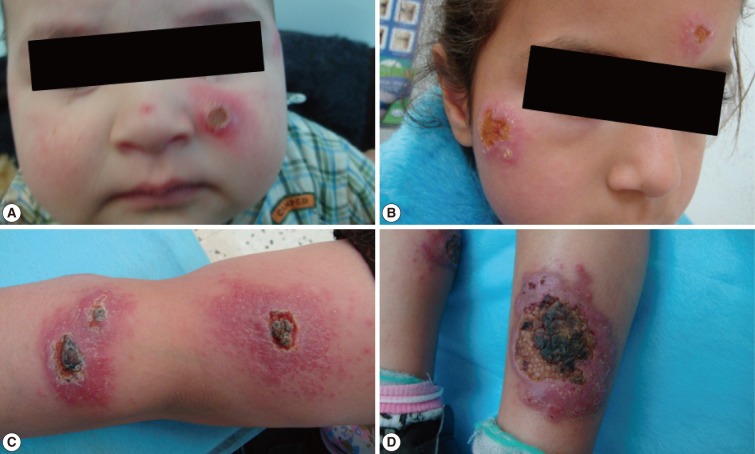
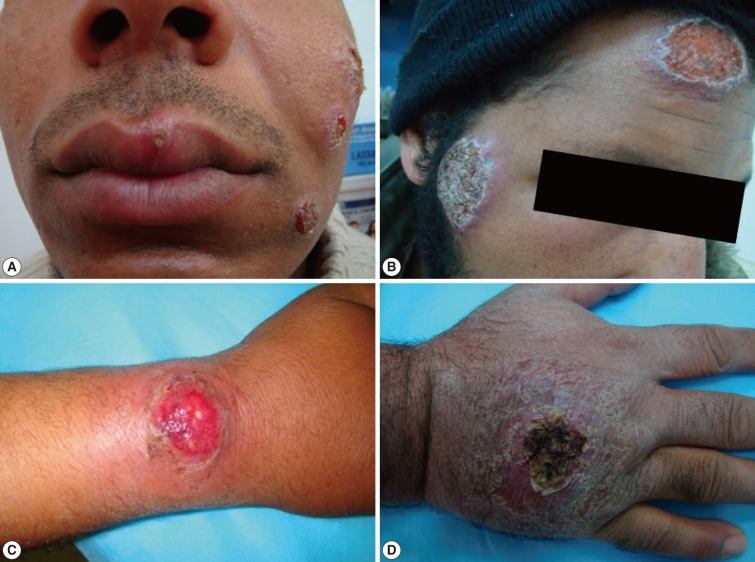
Fig. 3Different forms of cutaneous leishmaniasis lesions among children cases. (A) Typical single nodulo-ulcerated lesion on the cheek of a child with raised indurated border and central depression. (B) Two nodulo-ulcerated lesions on the forehead and cheek of a child. (C) Multiple lesions on the arm and forearm with necrotic tissue and plaque formation. (D) Two large ulcerated lesions on both legs with erythema, dark dry plaques and secondary infection.
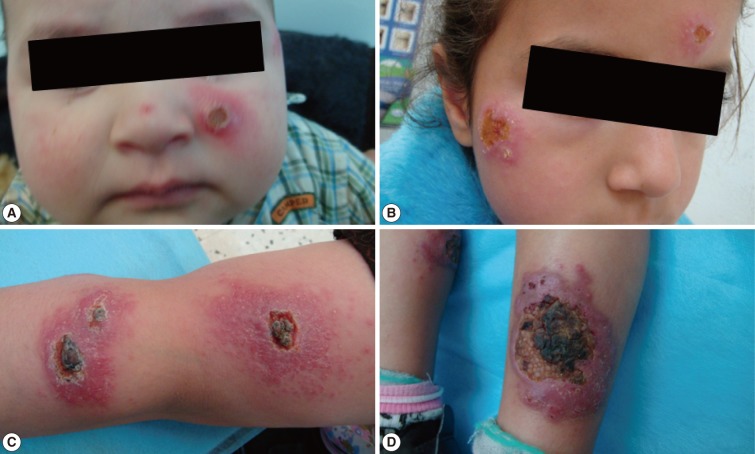
Fig. 4Different forms of cutaneous leishmaniasis lesions among adult patients. (A) Multiple lesions on the left side of an adult's face (the upper 2 are nodulo-ulcerated while the lower one is a papulo-ulcerated lesion). (B) Two large lesions (a nodulo-ulcerated lesion on the forehead and a crater-like one on a patient's cheek). (C) A single large nodulo-ulcerated inflamed lesion on the forearm. (D) A single crusted lesion on the dorsum of the hand with erythematous dry plaques.
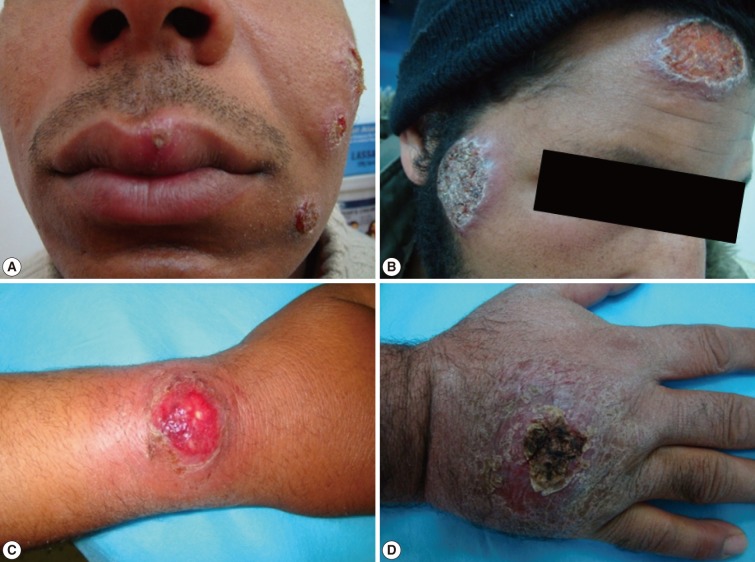
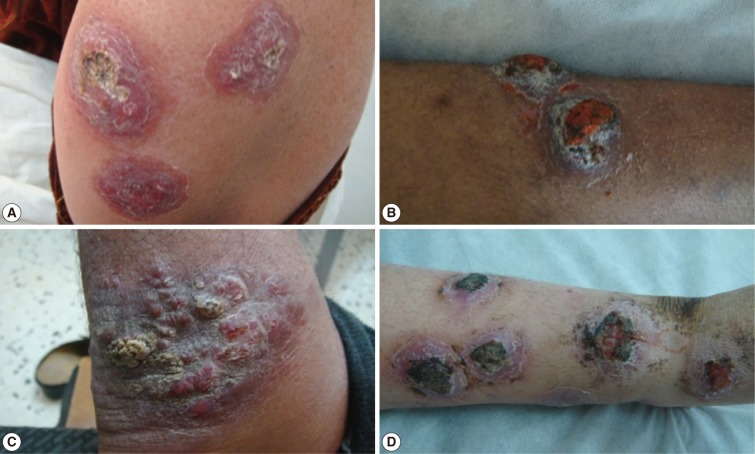
Fig. 5Different forms of cutaneous leishmaniasis lesions affecting various body sites of adult patients. (A) Multiple indurated ulcerated scaly plaque lesions on the forearm of an adult. (B) Two indurated ulcerated scaly plaque lesions on the leg of an adult. (C) Satellite form, crusted nodular lesions on the posterior aspect of an ankle joint. (D) Multiple ulcerated lesions on the leg of an adult with erythematous dry plaques, inflammation and secondary infection.
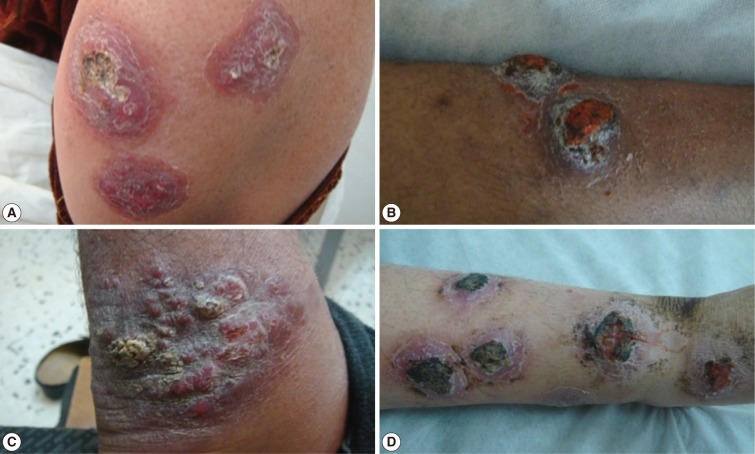
Fig. 6Age-group and gender distribution of the cutaneous leishmaniasis cases of Al-jabal Al-gharbi, Libya.
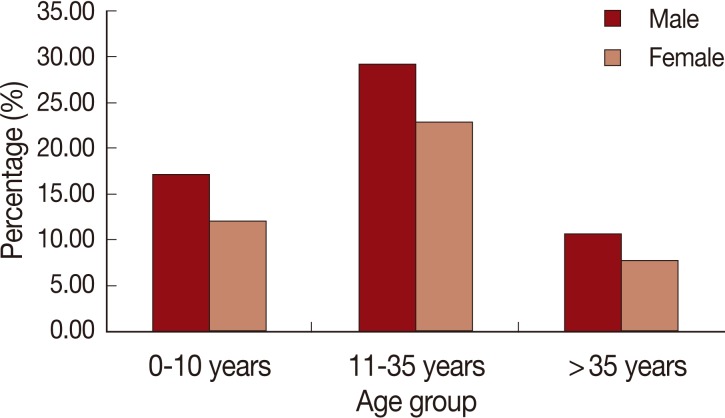
Table 1.Age and sex distribution of cutaneous leishmaniasis patients in Al-jabal Al-gharbi, Libya
Table 1.
|
Gender |
Male patients No. (%) |
Female patients No. (%) |
Total No. (%) |
|
Age group |
|
|
0-10 years |
24 (17.1) |
17 (12.2) |
41 (29.3) |
|
> 10 years |
56 (40.0) |
43 (30.7) |
99a (70.7) |
|
Total |
80b (57.1) |
60b (42.9) |
140 (100.0) |
Table 2.Distribution of cutaneous leishmaniasis (CL) cases among different subprovinces of Al-jabal Al-gharbi, Libya
Table 2.
|
Gender |
Male patients No. (%) |
Female patients No. (%) |
Total No. (%) |
|
Subprovinces |
|
|
Al-Kawasem |
19 (23.8) |
20 (33.3) |
39 (27.9)a
|
|
Bani Khalifa |
12 (15.0) |
11 (18.3) |
23 (16.4) |
|
Asabaa |
6 (7.5) |
6 (10.0) |
12 (8.6) |
|
Al orban |
7 (8.8) |
4 (6.7) |
11 (7.9) |
|
Rabtah |
11 (13.8) |
7 (11.7) |
18 (12.9) |
|
Taghasat |
7 (8.8) |
4 (6.7) |
11 (7.9) |
|
El-hera |
4 (5.0) |
4 (6.7) |
8 (5.7) |
|
Mezdah |
4 (5.0) |
0 (0.0) |
4 (2.9) |
|
Kekla |
2 (2.5) |
2 (3.3) |
4 (2.8) |
|
Zantan |
1 (1.3) |
2 (3.3) |
3 (2.1) |
|
Anser |
3 (3.8) |
0 (0.0) |
3 (2.1) |
|
Neismah |
1 (1.3) |
0 (0.0) |
1 (0.7) |
|
Elraianah |
1 (1.3) |
0 (0.0) |
1 (0.7) |
|
Jadu |
1 (1.3) |
0 (0.0) |
1 (0.7) |
|
Elgwalish |
1 (1.25) |
0 (0.0) |
1 (0.7) |
|
Total |
80 (100.0) |
60 (100.0) |
140 (100.0) |
Table 3.Occupational distribution of adult cutaneous leishmaniasis (CL) cases reported at Al-jabal Al-gharbi, Libya
Table 3.
|
Occupation |
No. of adult CL patients (%) |
|
Farm workers |
19 (26.0)a
|
|
Students "high school and university" |
18 (24.7) |
|
Housewives |
17 (23.3) |
|
Artisans |
5 (6.8) |
|
Teachers |
4 (5.5) |
|
Businessmen |
3 (4.1) |
|
Policemen |
3 (4.1) |
|
Pensioners |
3 (4.1) |
|
Nurses |
1 (1.4) |
|
Total |
73 (100.0) |
Table 4.Distribution of cutaneous leishmaniasis children cases according to school attendance at Al-jabal Al-gharbi, Libya
Table 4.
|
Educational stage |
No. of children cases (%) |
Z test |
P-value |
|
Preschool children |
18 (26.9) |
4.3 |
0.0001 |
|
School children |
49 (73.1) |
|
|
Table 5.Distribution of cutaneous leishmaniasis lesions (single or multiple) by sex among the reported children and adult cases at Aljabal Algharbi, Libya
Table 5.
|
Gender |
Male patients
|
Female patients
|
Total
|
|
Age group |
|
Single lesion |
Multiple lesions |
Single lesion |
Multiple lesions |
Single lesion |
Multiple lesions |
|
Children |
20 |
19 |
14 |
14 |
34 |
33 |
|
Adults |
14 |
28 |
11 |
19 |
25 |
48a
|
|
Total |
34 |
47 |
25 |
33 |
59 |
81 |
|
X2
|
1.99 |
0.37 |
3.90 |
|
P-value |
0.07 |
0.27 |
0.035 |
Table 6.Types of cutaneous leishmaniasis lesions (nodulo-ulcerated, Papulo-ulcerated, and indurated ulcerated scaly plaque) by sex among the reported children and adult cases at Aljabal Algharbi, Libya
Table 6.
|
Age group |
Children
|
Adults
|
|
Lesion type |
|
No. of males (%) |
No. of females (%) |
No. of males (%) |
No. of females (%) |
|
Nodulo-ulcerative |
21 (31.3)a
|
20 (29.9)a
|
22 (30.1)a
|
20 (27.4)a
|
|
Papulo-ulcerated |
17 (25.4)b
|
8 (11.9)b
|
16 (21.9)b
|
9 (12.3)b
|
|
Indurated ulcerated scaly plaque |
1 (1.5) |
0 (0.0) |
3 (4.1) |
3 (4.1) |
|
Total |
67 (100%) |
73 (100%) |
|
P-value |
0.001a and 0.001b
|
0.0001a and 0.002b
|
Table 7.Types of cutaneous leishmaniasis (CL) lesions (wet and dry) by sex among the reported children and adult cases at Aljabal Algharbi, Libya
Table 7.
|
Age group |
Children
|
Adults
|
|
Lesion type |
|
No. of males (%) |
No. of females (%) |
No. of males (%) |
No. of females (%) |
|
Wet CL |
38 (56.7)a
|
38 (56.7)a
|
38 (56.7)a
|
38 (56.7)a
|
|
Dry CL |
1 (1.5) |
1 (1.5) |
1 (1.5) |
1 (1.5) |
|
Total |
67 (100%) |
73 (100%) |
|
P-value |
0.0001 |
0.001 |
Table 8.Frequency distribution of the affected body sites of cutaneous leishmaniasis presented as comparisons between children and adults in both sexes among patients of Al-jabal Al-gharbi, Libya
Table 8.
|
Site of lesion |
Gender |
No. male patients (%)
|
No. male patients (%)
|
Total (%)
|
|
Child |
Adult |
Child |
Adult |
Child |
Adult |
|
Upper extremities |
12 (14.5) |
27 (27.6) |
10 (12.1) |
21 (21.4) |
22 (26.5) |
48 (48.9) |
|
Lower extremities |
11 (13.5) |
23 (23.5) |
9 (10.8) |
16 (16.3) |
20 (24.1) |
39 (39.8) |
|
Face |
24 (29.0) |
4 (4.1) |
14 (16.9) |
2 (2.04) |
38 (45.8) |
6 (6.1) |
|
Back |
2 (2.0) |
2 (2.0) |
1 (1.2) |
0 (0.00) |
3 (3.6) |
2 (2.0) |
|
Neck |
0 (0.0) |
2 (2.0) |
0 (0.0) |
1 (1.02) |
0 (0.0) |
3 (3.1) |
|
Total |
49 (59.0) |
58 (59.2) |
34 (41.0) |
40 (40.8) |
83 (100.0) |
98 (100.0) |
|
X2
|
23.9 |
14.4 |
38.3 |
|
P-value |
0.0001 |
0.001 |
0.0001 |
Table 9.Frequency distribution of cutaneous leishmaniasis patients according to their reports of having similar lesions in other family members and presence of sandflies and rodents in the areas of their residence at Al-jabal Al-gharbi, Libya
Table 9.
|
Patients’ answer |
Present No. (%) |
Absent No. (%) |
Z-test |
P-value |
|
Item |
|
|
Presence of rodents |
126 (90.0) |
14 (10.0) |
15.8 |
0.0001 |
|
Presence of sandflies |
131 (93.6) |
9 (6.4) |
21.1 |
0.0001 |
|
Presence of lesions in other members of the same family |
56 (40.0) |
84 (60.0) |
-2.42 |
0.007 |