The First Case of Capillaria hepatica Infection in a Nutria (Myocastor coypus) in Korea
Article information
Abstract
This study reports the first case of Capillaria hepatica infection in a nutria in Korea. Ten nutrias, captured near the Nakdong River, were submitted to our laboratory for necropsy. White-yellowish nodules were found in the liver of 1 of the nutrias at necropsy. Histologically, the lesions were granulomatous, and infiltrations of lipid-laden macrophages, eosinophils, and several multinucleated giant cells were observed. The lesions consisted of numerous eggs and necrotic hepatocytes. The eggs were lemon-shaped and had polar plugs at the ends of both long sides. The eggs were morphologically identified as those of C. hepatica. Worldwide, C. hepatica infection in nutrias is very rare. Nutrias are a kind of livestock, as well as wildlife; therefore, an epidemiological study for parasitic infections needs to be conducted.
Nutrias (Myocastor coypus) are carriers of a variety of zoonotic pathogens and parasites, and can transmit diseases to pets and livestock. They are susceptible to a number of infections, including rabies [1], equine encephalomyelitis [2], paratyphoid [3], salmonellosis [4], papillomatosis [5], leptospirosis and toxoplasmosis [6], richettsiosis [7], coccidiosis [8], and sarcosporidiosis [9]. The locations of the parasites in nutrias can vary; some found both internally and externally, just like the nematode Strongyloides myopotami. Other endoparasites discovered included 11 species of trematodes, 21 cestode species, 1 acanthocephalan, and 31 nematode species [10]. External parasites of nutrias include the chewing louse, fleas, and several tick species [11,12]. To date, only 2 cases of Capillaria hepatica infection have been reported in nutrias worldwide [13,14].
Due to the damages they cause and the potential transfer of diseases they can carry, nutrias are controlled and eradicated by the Ministry of Environment and local governments. Ten dead adult nutrias were sent to our laboratory, which were obtained from a baited wire cage trap in a wetland around the Nakdong River (N35°18'47.80" E128°47'16.83") during 12-13 March 2013. The sample set included 5 males and 5 females (2 of which were pregnant). The livers were collected at necropsy, formalin-fixed, and paraffin-embedded. Sectional specimens of 3 µm thickness were stained with hematoxylin and eosin. Basophilic mineralization was found in the bile ducts in 2 of the nutrias. In the liver of 1 nutria, there were multifocal granulomatous lesions, distributed randomly (Fig. 1). The infiltrating cells were predominantly lipid-laden macrophages, eosinophils, and several multinucleated giant cells. The lesions consisted of numerous eggs and necrotic hepatocytes. The eggs were lemon-shaped, unembryonated, and polar plugs occurred at the ends of both long sides (Fig. 2). Longitudinally sectioned parasites were found in a different lesion, which was mainly infiltrated by macrophages (Fig. 3). The eggs were identified as those of C. hepatica.
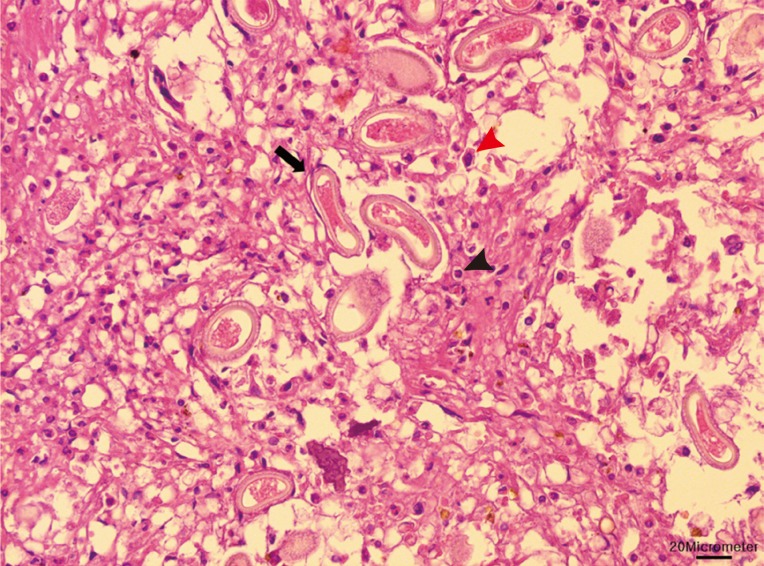
Macrophages (red arrowhead) and eosinophils (black arrowhead) infiltrated in the granulomatous lesion. Numerous eggs (black arrow) and necrotic hepatocytes were seen in the lesion. Bar=20 µm.

Higher magnification of the eggs showing lemon-shaped morphology with polar plugs occurring at the ends of both long sides. Bar=10 µm.

Longitudinal section of a nematode was found with infiltrations of lipid-laden macrophages in the liver. Bar=50 µm.
Nutrias, which are inhabits of South America, are a representative invasive alien mammal in Korea. In 1985, 100 nutrias were introduced to Korea from France, for use of their meat and fur. All of the nutria died due to the inappropriate breeding system used, so an additional group of 60 nutrias were brought from Bulgaria in 1987. By 2001, the number of nutrias had grown to 150,000; however, commercialization of nutrias had failed, and they were released into the wild. At present, the nutrias inhabit an area close to the Nakdong River Basin, and Jeju Island. Nutrias may cause damage, as a direct result of feeding and burrowing. They can also cause crop damage in areas where agricultural fields are located near aquatic habitats. Nutrias can burrow under and through water control structures, such as levees, dikes, and dams, weakening these structures. Their burrows can cause significant weakening and collapse of banks and road beds, particularly in locations where the soil is saturated, and the slope is >45 degrees [15].
The morphology of C. hepatica has already been described in detail in previous reports. C. hepatica is a filiform nematode. A typical adult C. hepatica is slender, with a narrow anterior part of the body, swelling gradually to the posterior part. Females measure 53-78×0.11-0.20 mm, whereas males are 24-37×0.07-0.10 mm [16]. In this study, the width of the parasite was 0.07-0.09 mm. The eggs of C. hepatica were oval in shape, averaging 54-65 by 29-33 µm in size, with a double shell. There were radiating striations in the outer shell, and polar plugs that did not protrude beyond the outer shell. In contrast, the eggs of Trichuris trichiura are elliptical, averaging 49-54 by 21-23 µm in size, have a double shell without radiating striations, and have polar plugs which protrude beyond the outer shell [17]. C. hepatica is a common parasite of rodents and many other kinds of mammals. It lodges into the hepatic parenchyma, where it lays eggs that remain trapped in the organ, but do not initially develop into the infective stage. The infected rodent must be eaten by carnivores, which digests and releases the eggs enclosed in the hepatic tissue, eliminating them through the feces into the external environment. When the infected eggs are consumed by rodents, the larvae are released into the intestine, enter the intestinal wall, and are carried through the bloodstream to the liver, where they mature in approximately 1 month [18].
During May-July 1978, a survey was carried out on the prevalence of C. hepatica among house rats and wild mice in 2 districts of Seoul, Korea. Approximately 38% of the 1,000 house rats examined were found to be infected [19]. Thereafter, Choe et al. [20] reported a case of massive hepatic infections by C. hepatica in a 14-month-old Korean girl with the symptoms of persistent fever, hepatomegaly, and leukocytosis with eosinophilia. This was the first human case of hepatic capillariasis reported in Korea. Diagnosis was made by liver needle biopsy, and scanning electron microscopic examinations of the biopsy specimen were performed. Thiabendazole therapy was initiated, but the patient developed liver disease-related IgA nephropathy during therapy [20].
In the liver of mountain gorillas (Gorilla gorilla beringei) infected with C. hepatica, many of the lesions were associated with the portal system, with a mild inflammatory reaction. The lesions consisted of an infiltrate of lymphocytes, giant cells, and fibrous connective tissue surrounding the eggs. In the center of the large lesion, necrosis and degenerating cells were present. However, there were no hepatitis lesions in the hepatic tissues between granulomatous lesions [21]. In our study, the lesion associated with the eggs of C. hepatica was also granulomatous, which was similar to other reports. This report is the first C. hepatica infection in nutrias in Korea. Since nutrias are a kind of livestock as well as wildlife, an epidemiological study for parasitic infections is necessary.
ACKNOWLEDGMENT
This study was supported by the Research Institute for Veterinary Science, College of Veterinary Medicine, Seoul National University, Seoul, Korea.
Notes
We have no conflict of interest related to this study.