Eosinophilic Pleuritis due to Sparganum: A Case Report
Article information
Abstract
Sparganosis is a rare parasitic disease caused by migrating plerocercoid tapeworm larva of the genus Spirometra. Infection in humans is mainly caused by the ingestion of raw or inadequately cooked flesh of infected frogs, snakes, and chickens. Here, we report a rare case of a 45-year-old man who was admitted to our hospital with left lower chest pain. The chest radiograph and computed tomography (CT) scan revealed localized pleural effusion in the left lower lobe; further, peripheral blood eosinophilia and eosinophilic pleural effusion were present. Percutaneous catheter drainage was performed, which revealed long worm-shaped material that was identified as a sparganum by DNA sequencing. The patient showed clinical improvement after drainage of the sparganum. This study demonstrates the importance of considering parasitic diseases in the differential diagnosis of eosinophilic pleural effusion.
INTRODUCTION
Sparganosis is a rare parasitic disease caused by migrating plerocercoid tapeworm larva of the genus Spirometra [1]. Although it has been reported worldwide, the major endemic areas are China, Japan, Taiwan, Korea, Vietnam, Thailand, and other South Asian countries [2,3]. Infection in humans occurs mainly by ingestion of raw or inadequately cooked flesh of infected frogs, snakes, and chickens [2]. Spargana migrate mainly to muscles or subcutaneous tissues. In rare occasions, they also migrate to the brain, spinal cord, thoracic cavity, or urogenital organs [2]. Here, we report a rare case of a patient with pleural sparganosis and no other infection that was initially misdiagnosed as parapneumonic effusion.
CASE RECORD
A 45-year-old man, who had visited a clinic 3 days prior with left lower chest pain for 2 weeks, was transferred to our hospital for left pleural effusion on chest radiography. He also presented with a total body skin rash, cough, sputum production, abdominal discomfort, and a febrile sense for 1 week. He did not report any specific past medical history. His personal history included current smoking (60 pack-years), social drinking, and an unusual habit of occasional frog and snake consumption for good health from the age of 25 years.
He presented with an acute ill-looking appearance and a body temperature of 38.6℃. Initial laboratory test showed a mildly increased white blood cell count of 12,000/µl with eosinophilia (15%). Blood chemistries were within normal limits. ELISA or immunofluorescence test was used for detecting specific antibodies in serum against Paragonimus westermani, Clonorchis sinensis, and Toxoplasma gondii, and the results were negative. The chest radiograph and CT scan revealed loculated pleural effusion in the left lower lobe (Fig. 1A, B).

(A) A chest radiograph of this patient showing pleural effusion in the left lung. (B) A chest computed tomography (CT) scan showing loculated pleural effusion with mildly enhanced pleura.
Thoracentesis revealed yellowish pleural effusion with an increased eosinophil count (25%). The specific gravity of the pleural effusion was 1.02 with 5.3 g/dl protein and 1.05 IU/L lactic dehydrogenase. The levels of adenosine deaminase (15.1 IU/L) and carcinoembryonic antigen (2.5 ng/ml) were within normal ranges. No malignant cells were detected on cytological examinations of the pleural effusion. We performed an evaluation to identify the etiology of the eosinophilic effusion and administered antibiotics for a possible hidden infection that may have caused the fever. Additionally, we decided to drain the loculated pleural effusion by percutaneous catheter drainage (PCD).
On the second day of PCD insertion, we found a long worm-shaped material in the drain bottle that had the appearance of yarn and an organism that was very long (about 70 cm). The organism was sent to the Parasitology Clinic for identification (Fig. 2A, B). As we had already tested for other parasites using ELISA, we did not consider sparganum as a possible cause, even when we saw the worm-shaped material. At this time, we used the PCR amplification for the mitochondrial cytochrome c oxidase 1 (cox1) target fragment. The PCR amplification and direct sequencing of the cox1 target fragment (353 bp in length corresponding to the positions 769-1,121 bp of the cox1 gene) were performed using the total genomic DNA extracted from this specimen. The cox1 sequences (353 bp) showed 98.8% (349/353) similarity to the reference sequences of the Japanese origin Spirometra erinaceieuropaei (GenBank no. AB-278575.1) and 90.6% (320/353) similarity to the reference sequence of the Japanese origin Spirometra proliferum (GenBank no. AB015753. 1) [4]. Thus, the organism was identified as a sparganum. Following that, we identified another 33-cm long sparganum through PCD. The patient was diagnosed with eosinophilic pleuritis caused by at least 2 spargana worms.
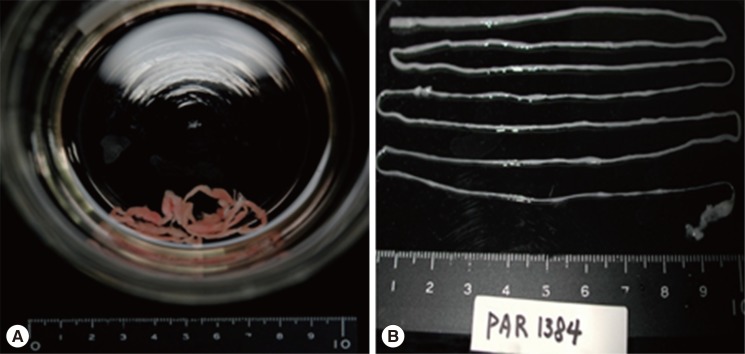
(A) Long worm-shaped material in the drain bottle that had the appearance of yarn and an organism. (B) The organism, confirmed to be a sparganum (plerocercoid of Spirometra erinacei), recovered which was approximately 70 cm long.
After discontinuation of antibiotic administration, the patient was treated with praziquantel in consideration of the possibility of other remaining trematode or cestode infection. The fever and peripheral eosinophilia showed a gradual decrease. After 1 week, he was discharged with improvement. At an outpatient follow-up 1 month later, he presented with normal peripheral eosinophilia and a complete clinical recovery.
DISCUSSION
Patrick Manson reported the first case of sparganum in a Chinese patient in 1882, and Uemura [2], a Japanese physician, reported a case of sparganum in 1971 that affected a Korean farmer's leg.
The parasite is transmitted to humans in 3 different ways: by ingestion of drinking water contaminated with copepods [5]; consumption of raw meats of 1 of the second intermediate hosts, such as frogs or snakes for medicinal purposes; and consumption of raw meats of frogs or snakes for food. There have been previous reports of humans being infected due to consumption of raw meats of second intermediate hosts, such as frogs or snakes [2]. Also in this case, the patient consumed these meats for medicinal purposes.
The sparganum grows slowly at the final destination. The clinical presentation of sparganosis varies according to the infected site; the common sites are the abdomen and lower extremity, and some rarely involved sites are the orbit, brain, spinal cord, and lungs [6]. The thoracic cavity is a rare site for localization of this parasite in humans, and reports of sparganosis with pleural effusion are rare in Korea. Previously, a study conducted in Thailand reported 34 cases of human sparganosis, with only 1 case that presented with pleural effusion [7]. The case in Thailand was different from ours in that the pleural effusion was accompanied by lung cancer. In addition, there was 1 report on a case of eosinophilic pleural effusion in Japan. Kamiya et al. [8] reported a similar case as ours. However, they used multiple-dot ELISA method differently. Sparganosis, in most cases, is diagnosed by biopsy [9]. Choi et al. [10] identified serologic reactions against antigenic proteins of spargana in sparganosis patients. SDS-PAGE and immunoblot analyses revealed that the antigenic proteins appeared to be specific-sized protein bands (29 kDa and 36 kDa) [10], which can be used for identification of sparganum infection. Therefore, in our case, the diagnosis of the sparganum was made based on immunoblot findings of specific protein bands that reacted with the patient's serum.
We administered praziquantel to our patient in spite of no evidence of other trematode or cestode infections. The effect of praziquantel in killing spargana is known to be none or negligible. Fever and peripheral eosinophilia in our patient decreased gradually. Although sparganosis is a rather rare etiology for eosinophilic pleuritis, it should be considered in cases with negative results of common parasite examinations and in cases with a history of raw meat ingestion, such as that of frogs and snakes. It is important to educate the general population regarding the avoidance of ingestion of raw frogs and snakes. Herein, we report a case of eosinophilic pleuritis caused by sparganum in a man with a history of frog and snake consumption for medicinal purposes.
Notes
We have no conflict of interest related to this report.