Recent Advances in Toxoplasma gondii Immunotherapeutics
Article information
Abstract
Toxoplasmosis is an opportunistic infection caused by the protozoan parasite Toxoplasma gondii. T. gondii is widespread globally and causes severe diseases in individuals with impaired immune defences as well as congenitally infected infants. The high prevalence rate in some parts of the world such as South America and Africa, coupled with the current drug treatments that trigger hypersensitivity reactions, makes the development of immunotherapeutics intervention a highly important research priority. Immunotherapeutics strategies could either be a vaccine which would confer a pre-emptive immunity to infection, or passive immunization in cases of disease recrudescence or recurrent clinical diseases. As the severity of clinical manifestations is often greater in developing nations, the development of well-tolerated and safe immunotherapeutics becomes not only a scientific pursuit, but a humanitarian enterprise. In the last few years, much progress has been made in vaccine research with new antigens, novel adjuvants, and innovative vaccine delivery such as nanoparticles and antigen encapsulations. A literature search over the past 5 years showed that most experimental studies were focused on DNA vaccination at 52%, followed by protein vaccination which formed 36% of the studies, live attenuated vaccinations at 9%, and heterologous vaccination at 3%; while there were few on passive immunization. Recent progress in studies on vaccination, passive immunization, as well as insights gained from these immunotherapeutics is highlighted in this review.
INTRODUCTION
Toxoplasmosis is a zoonotic disease caused by the obligate intracellular parasite Toxoplasma gondii. Toxoplasmosis can cause severe neurologic, ocular, and systemic diseases in neonates and individuals with weakened immune system [1]. In immunocompetent adults, T. gondii infection is usually asymptomatic and forms dormant tissue cysts containing bradyzoites at immune-privileged sites such as the brain and muscles [1]. When immunity is impaired, latent infection is then reactivated causing widespread tissue destruction and severe pathology, and is among the major causes of mortality in AIDS patients. Disease severity is exacerbated in underdeveloped countries with limited financial resources and infrastructure to acquire and distribute the anti-retroviral drugs [2]. The sub-Saharan African continent is a dire example of this. A high rate of HIV infection combined with high seroprevalence of T. gondii co-infection would result in an estimated 2.5 to 10 million people in this region who are at risk of dying from toxoplasmic encephalitis [3]. Atypical strains, predominantly found in South and Central America, have caused debilitating diseases, especially in the form of recurrent ocular disease that can lead to blindness in otherwise healthy adults [4,5]. This is an especially critical problem in Brazil where the current risk of ocular toxoplasmosis is at a high rate of 18% [6]. Thus, this pathogen remains as a severe public health problem in developing countries.
The development of a vaccine against toxoplasmosis is highly important to arrest the spread of the disease because in theory, a single treatment confers life-long protective immunity. Significant strides have been made in the past 15 years in antigen isolation and characterization, gene-cloning, generation of parasite mutants, and immunological methods [7]. Vaccine studies demonstrating robust disease control are antigens that could elicit a protective Th1-biased immune response and generation of long-lived cytotoxic CD8+ T cells producing IFN-γ [8]. Vaccinations have thus far proven effective at reducing disease mortality and parasite cyst burden in mice, but do not achieve complete protection. Thus, sterile immunity from the disease remains an elusive target.
As toxoplasmosis is an opportunistic infection causing illness in immunocompromised patients with reduced cellular immunity, therapeutic intervention to reduce pathogenesis is also needed. Current anti-parasitic drug treatments cause toxic hypersensitivity reactions and are teratogenic, presenting a need for safer and well-tolerated treatment options. As the major cause of toxoplasmosis mortality is due to reactivation of latent infection, while considerable morbidity can result from the tendency of congenital infections to relapse with retinal damage, studies on passive immunization to decrease parasitemia burden is necessary for limiting the disease pathology. The aim of this review is to focus on recent advances in both the active and passive immunization studies against toxoplasmosis in murine models.
VACCINES
Based on observations that chronic infection is able to confer immunological memory to pregnant mothers, normally preventing disease transmission to fetuses even if re-exposed during gestation, a toxoplasmosis vaccine seems highly feasible. Various vaccination strategies against toxoplasmosis in rodent models have been employed in the past decades, ranging from inactivated vaccines, protein vaccines in subunit or multi-antigenic cocktails, and DNA vaccines. However, efficacious vaccines remains a challenge as vaccine experiments have not been able to confer sterile immunity against infection. Sterile immunity is a protection endpoint that is assessed not only by survival rate, but also by the lack of tissue cysts after challenge infection, which is a hallmark of chronic infection. Only a few live, attenuated strains of T. gondii have been able to elicit a potent Th1 immune response for effective disease resistance, but live vaccines such as the commercially-licensed ToxoVax® (Intervet B.V.) used for sheep immunization is considered unsafe for human use and is restricted in its applications due to safety concerns as well as a short shelf-life.
It has been established that the IFN-γ-secreting CD8+ T lymphocyte is the major T cell subset involved in crucial long-term protective immunity in toxoplasmosis [9,10]. Thus, an ideal vaccine to treat toxoplasmosis should be sufficiently immunogenic to induce a Th1-type immune response to control disease pathogenesis, as well as to prevent the development of chronic tissue cysts. There are several challenging features in toxoplasmosis vaccine development. First is the stage-specific expression of parasite proteins which may render vaccines targeted to the acute stage ineffective at the chronic stage and vice versa. Second is the presence of varied T. gondii genotype strains means that vaccinations against a particular strain may not be protective against infections with other strains, especially with growing concern over the virulent, atypical strains from South America [11]. Third is the parasite's innate immune evasion mechanisms that enable the sequestration of dormant tissue cysts as a life-long chronic infection has been recalcitrant to both drug treatments and vaccination attempts to eliminate infection.
PROTEIN VACCINES
Significant advances made in the past decade in understanding the cell and molecular biology of the T. gondii parasite has provided the impetus to the current focus of vaccines based on defined subcellular components of the parasite. Among the parasite antigens that have been researched extensively in vaccination experiments are the surface antigen glycoproteins, or SAGs. Notably, investigations on highly immunogenic SAG1 expressed on tachyzoites have repeatedly yielded high survival rates and significant reduction in brain tissue cysts load [reviewed in 7]. SAG1 immunization studies by Khan et al. [12] and Debard et al. [13] demonstrated 100% and 85% reduction in brain cysts load when adjuvanted with QuilA and cholera toxin (CT) respectively. In a move to use less toxic adjuvants, a SAG1 vaccine co-administered with non-toxic heat-labile enterotoxin (LT) also provided significant but slightly lowered protection and a 78% reduction in brain cyst loads following intranasal delivery [14]. However, this highlights the need to develop adjuvants to simultaneously maximize immunostimulatory efficacy and safety. A recent development was the use of saponins from Panax ginseng roots as a non-toxic adjuvant in a ROP18 vaccine formulation, which improved protective efficacy of the vaccine [15]. Thus, these ginseng roots that have been used as traditional medicine hold potential in enhancement of the anti-toxoplasmosis immune responses and could be investigated further in vaccine formulations.
Immunity against T. gondii is highly complex and studies have shown how differences in host genetic background can influence vastly divergent disease outcome. This was apparent in a SAG1 vaccination which proved inhibitory to the materno-fetal transmission of toxoplasmosis in BALB/c mice by 50%, but not with CBA/J mice, which in fact exhibited increased disease transmission to fetuses [16]. As congenital toxoplasmosis can cause severe damage to a baby's brain, eyes, and nervous system, and current drug treatments are teratogenic, immunization efficacy in limiting congenital transmission should be also considered an important endpoint in more vaccine research.
In recent years, vaccination with several protein antigen combinations resulted in increased survival rates in mice following lethal challenge with type I parasite. The most significant gains in survival rates were reported for vaccine antigen SAG1 (80%) [17], SAG1+SAG2 (83%) [18], TgACT (50%) [19], and TgRACK-1 (45%) [20]. Among these findings, the unique study by Chuang et al. [17] used poly (lactide-co-glycolide) (PLG) microparticle encapsulation of the SAG1 and SAG2 protein antigens as a sustained-release vaccine formulation, which is an attractive concept for inducing prolonged disease immunity. Overall, with proper optimization to prevent antigens from denaturing during the encapsulation process, this is a strategy which could be also applied in various other immunotherapeutics formats to maintain therapeutic levels in blood or tissues for extended periods of time.
An important endpoint in the evaluation of toxoplasmosis vaccines is the provision of sterile immunity from the cystic bradyzoite stage, which is recalcitrant to current drug treatments and the source of disease reactivation in chronically-infected individuals. Thus, scoring the effects of immunization on brain tissue cysts loads in mice model gives crucial insights on vaccine utility. In lieu of this, the parasite antigens that have been included in numerous multi-antigen vaccines with good success are the ROP2 and GRA4 proteins. ROP2 is an abundant rhoptry protein essential in parasite multiplication and host invasion [21]. GRA4 is a dense granule protein localized to the intravacuolar network of the parasitophorous vacuole (PV) and involved in nutrient acquisition [22]. Both antigens have shown Th1-type immunogenicity, and its protective efficacy in various mouse strains [23] indicates these antigens as vaccine candidates that warrant further studies. The highest reductions in brain tissue cyst loads conferred were through the immunization with protein antigen combinations of ROP2+ROP4+SAG1 (90%) [24], ROP2+ROP4+GRA4 (84%) [25], and GRA7 nanoparticles (72%) [26]. In particular, the GRA7 nanoparticle is a novel vaccine based on monomeric T cell epitopes of GRA7 protein that self assembles into nanoparticles similar to a viral capsid. This nanoparticle vaccine is self-adjuvanting and demonstrates partial protective immunity to both type I and type II challenge infections.
Another interesting development was the vaccination with a recombinant plant-produced GRA4 antigen [27]. While a plant-produced toxoplasmosis antigen is not novel in itself [28], this work done by del L. Yácono et al. [27] involved the oral immunization of each mouse with leaf extracts equivalent to approximately 0.5 µg of GRA4 antigen. The substantial reduction in tissue cysts (59%) achieved by a low-dose vaccine followed by challenge with the cyst-forming type II strain potentiates the GRA4 antigen as a strong vaccine candidate. As T. gondii parasites mainly gain host entry by the intestinal mucosa route, an oral vaccine that can be potentially produced at lowered cost in plants shows good prospects. A summary of some of the most recent studies in protein vaccines against toxoplasmosis is provided in Table 1.
DNA VACCINES
DNA vaccination has the distinct advantage of being capable of generating an effective immune response with substantial cost benefits. DNA vaccinations against toxoplasmosis would generally induce an enhanced Th1 immune response with an increased production of inflammatory cytokines IFN-γ and IL-2 to limit infection. An analysis across T. gondii DNA vaccination studies reveals that the inducement of a Th1-type cellular immune response with significantly elevated levels of IFN-γ and IL-2 does not correlate with the degree of protective outcomes in mice. This means that the level of immunogenicity is not necessarily predictive of survival rates, as can be appreciated in several recent examples [33,34,35,36]. However, DNA vaccinations in general has shown to be effective at inducing the activation and proliferation of CD4+ and CD8+ T cells, along with eliciting specific antibodies essential for control of chronic infection.
The drawback of DNA vaccines is that the immune responses it generates are usually too weak to be significantly protective when administered in higher primates and humans [37]. Improvement in its potency can be gained by strategies such as codon optimization for increased protein expression, co-expression of cytokines, and molecular adjuvants, and using a heterologous prime-boost strategy [7,38]. In addition, DNA vaccination strategies using a combination of multiple antigens have been shown to be more effective in enhancing protective immunity than single subunit antigens [reviewed in 11]. A case in point is the antigenic combination of micronemal proteins (MIC2, MIC3, MIC4, M2AP, and AMA1) which achieved significant protection of 84% reduction in the brain tissue cyst burden and longer survival durations than single subunit antigen vaccination [39]. To ensure immunogenicity of the vaccines, the authors formulated a DNA vaccine based on known T-cell epitopes instead of using full-length antigens. This highlights that the identification of immunogenic epitopes capable of eliciting humoral and cellular immune responses is another important parameter to consider in vaccine design. The in silico identification of these functional epitopes seems increasingly possible in recent times via predictive binding algorithms for humans' MHC I molecules [8, reviewed in 40].
Co-administration of adjuvants has also shown to be a useful approach in boosting the immune response in DNA vaccinations to therapeutic levels. A recent example of this is the MIC8 vaccination studies. The MIC8 gene previously garnered a lot of interest due to the completely defective invasion phenotype of MIC8-parasite knockout mutant [41]. However, DNA vaccination with MIC8 alone generated only a modest increase in survival time [42]. In a strategy to enhance the protective immunity from MIC8 vaccination, co-administration of mIL-21 and mIL-15 cytokines showed improved survival time from 10.3±0.9 days (without cytokines) to 16.2±1.3 days (with cytokines) [43].
The highest protection achieved with DNA vaccination against toxoplasmosis was obtained by immunization with a plasmid encoding SAG, which conferred 100% survival in CH3 mice and 80% survival in BALB/c mice for at least 6 months post-lethal challenge [44]. The potency of this protective immunity exceeded a similar experiment using SAG1 protein vaccine which prolonged mouse survival, but couldn't prevent complete mortality [45]. However, several more recent studies using either the SAG1 antigen alone or in combination with other antigens showed less robust protective immunity, where the highest survival rate was 40%, even with the use of the same strain of parasite and mouse host [46,47,48]. This ambiguity may be attributed to the use of different SAG1 epitopes in each study. Another immunization study which also demonstrated a significant survival rate of 100% and 40% in BALB/c and C57BL/6 mice respectively following a lethal challenge, used a multi-epitope DNA vaccination [49]. This multi-epitope vaccine comprising 6 antigenic epitopes from SAG1, GRA1, GRA2 and GRA4 antigens also showed effective stimulation of a protective Th1 immune response; and forms part of an accumulating body of evidence on the efficacy of multi-antigen vaccinations against a complex parasite such T. gondii [49,50,51].
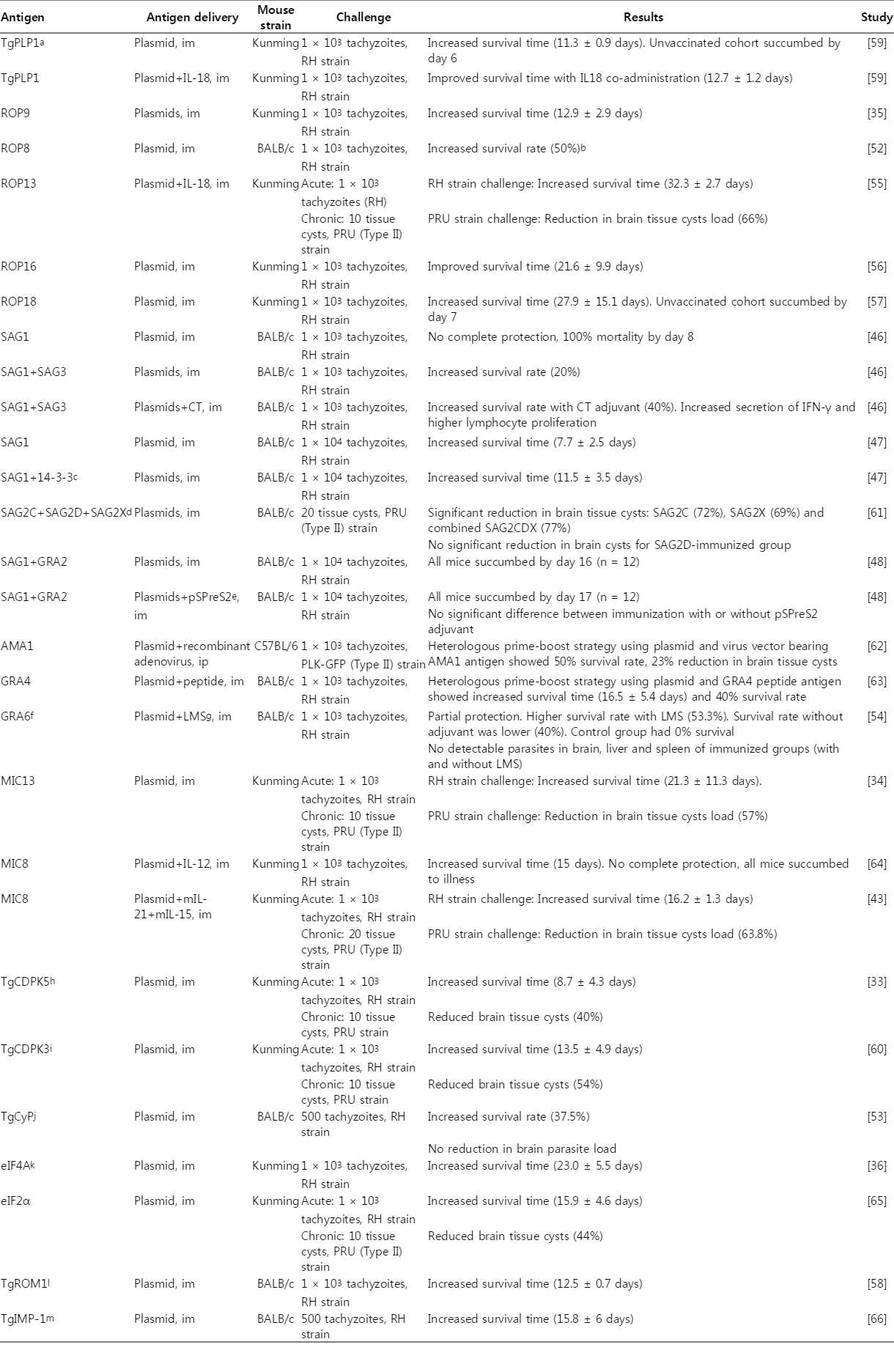
Over the past 4 years, toxoplasmosis DNA vaccination studies expanded to the use of many new parasite antigens, several of which yielded promising protective outcomes (Table 2). Among these latest developments, several single-antigen vaccines were successful in producing survivors from a lethal infection challenge, such as ROP8, TgCyP, and GRA6 with a range of survival rate between 37.5-50.0% [52,53,54]. Another subset of these DNA vaccine studies produced a significant increase in survival time (≥3 weeks) but no survivors following a lethal challenge, which was the ROP13, ROP16, ROP18, MIC13, and eIF4A [34,36,55,56,57]. There was a less significant survival outcome (≤2 weeks) from yet another subset of antigens, such as TgROM1, TgPLP1, TgCDPK3, and TgCDPK5 [33,58,59,60]. For effective comparisons, these recent studies in DNA vaccinations against toxoplasmosis are summarized in Table 2. Taken together, the next generation of vaccine design could include the empirical evaluation of functional antigenic epitope combinations in conjunction with immunological adjuvants for maximal efficacy.
LIVE, ATTENUATED VECTORS
Another vaccination strategy against toxoplasmosis involves the use of live, attenuated vectors, such as viruses and bacteria as vehicles to express recombinant T. gondii antigens in the host. The use of a live, attenuated vector to deliver T. gondii antigens provides the benefits of mimicry of the intracellular niche of the parasite by using innocuous recombinant virus or bacteria, and has been shown to be able to induce complete protection in some instances due to its intrinsic adjuvant properties. An example of this was shown in a DNA/viral vector heterologous prime-boost vaccination experiment. The GRA4 antigen delivered via recombinant attenuated vaccinia virus afforded 100% survival from infection with type II PLK/GFP tachyzoites, no detectable brain tissue cysts on 45 days post-infection, and significantly reduced brain parasite load [67]. In contrast, the plasmid-plasmid DNA vaccination group showed a lower survival rate of 70%. Some of the live vectors used in T. gondii vaccination studies include Salmonella typhimurium, pseudorabies virus, adenovirus, and modified vaccinia Ankara (MVA) (Table 3). The major advantages of live vectors as vaccine vehicles are its prospect as oral vaccines leading to inducement of mucosal immunity, as well as the potent immune response it elicits. These advantages has led to the development of these live vaccine formats for diseases ranging from cholera [68], to HIV infection [69] and cancer [70]. However, the use of recombinant, live vaccines faces the inevitable obstacles of safety risks and genetically-modified organisms (GMO) regulatory issues. The safety concerns that need to be addressed other than the more obvious ablation of virulence, is the lowering of risk of autoimmune responses and non-administration to immunocompromised individuals [reviewed in 71]. The vaccination studies using live, attenuated vectors against toxoplasmosis are summarized in Table 3.
Protective immunity to toxoplasmosis is highly complex as the interplay between the parasite's strain type and the host's genetic background influences disease pathogenesis [76,77]. The host's genetic influence over the selective processing of antigenic peptide, with the subsequent ability to present certain peptides determines disease severity in each individual. Therefore, the challenge of vaccine design is to determine protective epitopes across the antigenic diversity in T. gondii strains, capable of engaging with the diverse MHCs encompassing a majority of the human population to produce protective immunity [40]. Vaccine researchers now have an additional resource for testing vaccines by referring to the toxoplasmosis infection and pathogenesis model developed by Dubey et al. [78]. This in-depth study details infection responses across different mouse strains and various T. gondii strains, including the atypical strains. Thus, this model can be a highly useful tool in testing vaccine efficacy by providing a foundational standard for comparison.
In considering the platforms for vaccine delivery from the myriad of strategies ranging from DNA to live vaccines, it is worth noting that each platform presents its own benefits and disadvantages that are beyond the scope of this article to delve into. The interested reader is referred to an excellent review by Bruna-Romero et al. [79] on this topic. The development of pre-emptive vaccines remains a highly important pursuit in light of the persistent nature of chronic toxoplasmosis that is refractory to current drug treatments, and the increasing mortality associated with reactivated infections.
PASSIVE IMMUNIZATION
In the acute stage of toxoplasmosis, the host's innate immune response plays a key role in the control of infection [80], while long-term protective immunity is mediated by the adaptive immune response that drives it into a quiescent chronic stage but does not completely eradicate the parasite [81,82]. The persistent chronic stage can be lethal in reactivated infections affecting immunocompromised hosts. Thus, passive immunization could be a useful means of limiting clinical disease in hosts with reduced cellular immunity.
A major cell subset critical in disease control is the CD8+ T cells which produces the cytokine IFN-γ [9,10]. It was revealed that the adoptive transfer of immune CD8+ T cells to naïve animals could confer protective immunity and prolong survival in acute infections [82]. Although these are encouraging findings, translation of the application of syngeneic cellular immunotherapeutics to the masses could be challenging. Nonetheless, studies on passive cellular immunization could still provide invaluable insights on the factors affecting long-term immune control of toxoplasmosis. The inbred C57BL/6 mouse strain is mostly used in such studies due to its susceptibility to succumb to chronic toxoplasmosis, even with avirulent strains. This condition mimics reactivation of latent infections arising from immune compromise. One of the recent and interesting findings of this is that adoptive transfer of immune CD8+ T cells to pre-recrudescent chronically-infected C57BL/6 mice could provide transient protection from disease reactivation for up to 4 weeks post-treatment [83]. However, this same treatment could not rescue endogenous CD8+ T cells from functional exhaustion leading to apoptosis and the impairment of memory T cells development. Thus, long-term protective immunity was not achieved. These results seem to suggest that adoptive cellular immunotherapy provides significant protection in acute toxoplasmosis, but not so in a model of disease reactivation in chronic toxoplasmosis.
While CD8+ T cells and IFN-γ are important mediators of the host immune responses to resist T. gondii infection, antibodies produced by B cells are essential for long-term resistance to the disease by the production of specific antibodies [84,85]. The absence of antibody production in the host exacerbates infection and results in chronic stage mortality. In a study using CD4+ depleted mice to parallel CD4+ deficiency in AIDS patients and other immunocompromised individuals, passive immunization with immune serum has shown to be able to prolong survival and transiently overcome impaired disease resistance [86]. This suggests that the production of specific antibodies together with cellular immune responses is an important feature in the generation of adaptive immunity to T. gondii. Therefore, the potential development of antibodies as immunotherapeutic agents to arrest disease progression would prove highly beneficial to individuals suffering from impaired immunity.
In an experiment using Fab antibody fragments specific to T. gondii SAG1 antigen, it was shown that the antibodies blocked parasites attachment to host cells in vitro by 52% at an antibody concentration of 200 µg/ml [87]. Passive immunization of mice with 10 mg of this antibody provided improved survival rates of up to 50% following lethal challenge infection [87]. This survival rate improvement is a significant finding considering that the Fab fragments used in this study is lacking the Fc region of full antibodies which mediates Fc-receptor-dependent phagocytosis of opsonized tachyzoites, cellular cytotoxicity and complement activation. Therefore, it would seem that the antibodies mediate its protective function by binding to the parasites' surface and directly blocking infection of host cells, which is consistent with an observation by Sayles and co-workers in their study [85].
Passive immunization with anti-GRA2 and anti-GRA6 monoclonal antibodies have also resulted in inhibition of parasite invasion and enhanced survival times in mice. However, the anti-SAG1 antibody yielded a more significant protective immunity than the antibodies targeted to the dense granules antigens [88]. Another passive immunization experiment using monoclonal antibodies against nucleoside triphosphate hydrolase-II (NTPase-II) showed no inhibition of parasite invasion, but a significant reduction in T. gondii replication and promoted prolonged survival in mice after a lethal challenge [89]. Taken together, the development of anti-toxoplasmosis antibodies providing greater protection from host infection could benefit from a combinatorial antibody formulation that blocks parasite attachment, invasion and proliferation; and thus warrants further investigations.
Recently, polyclonal antibodies against T. gondii was produced in egg yolks from immunized chicken and showed a comparable avidity and reactivity profile to mammalian-derived antibodies [90]. The main advantage of using a chicken platform is its' very high yield of antibodies (4 mg/ml purified IgY) which can be purified inexpensively and obviates the need to bleed animals. Because of the phylogenetic distance between avian and mammalian species, chicken IgY antibodies has the inherent biochemical advantage of not activating the mammalian complement system, and does not react with rheumatoid factors and anti-human IgG antibodies [91]. However, the therapeutic effect of these avian antibodies remains uncertain and requires further study. In conclusion, regardless of the production platform for anti-toxoplasmosis antibodies, therapeutic antibodies to ameliorate the significant disease morbidity and mortality associated with reactivated infections is obviously needed and evidence from past studies shows that this can be a feasible toxoplasmosis treatment for immunocompromised patients.
CONCLUDING REMARKS
The immune evasive nature and complex life cycle of T. gondii has made the design of immunotherapeutics aimed at providing complete protection a daunting challenge. Generally, vaccine studies thus far have shown that multi-antigenic formulations confer better protection than single-subunit vaccines. At this juncture, research on adjuvants for vaccine co-administration is also crucial as alum has been the only one out of five licensed human adjuvants tested with toxoplasmosis vaccine formulations. In context of toxoplasmosis vaccination, this isn't an ideal option as alum augments the Th2 immune response, instead of the predominantly Th1 responses usually seen in protective immunity. Therefore, studies on multiple combinations of antigens with co-delivery of adjuvants or cytokines may yield better protective outcomes and progressively narrow the gap towards achieving sterile immunity. Vaccines and immunotherapeutic antibodies targeted to the parasite's surface membrane antigens present highly promising prospects considering the high immunogenicity of these antigens and the resulting significant parasitaemia inhibition from multiple studies reviewed thus far. Therefore, immunotherapeutics targeting either a single or multiple parasite surface antigens, in combination with invasion-related antigens such as the MIC, AMA1 and ROP proteins can be a potential strategy for generating a neutralizing immune response against toxoplasmosis. The discovery of atypical strains in the South American region which are more virulent than the classic European types I, II and III strains, and causes severe illness; would mean that next generation research efforts should also consider designing assays for cross-protection evaluation. These research efforts could be aided by a presently available model of toxoplasmosis infection strains in mouse [78]. A universal or multi-type anti-T. gondii immunotherapeutics is a highly valuable goal for global disease control. To this end, the completion of the genomes of predominant strains of T. gondii together with bioinformatics algorithms to predict MHC I-binding peptides could be a valuable tool to inform the design of broadly protective immunotherapeutics.
ACKNOWLEDGMENTS
Work related to the preparation of this review was supported by University of Malaya, Malaysia (grant: UM.C/625/1/HIR/MOHE/SCI/18).
Notes
We have no conflict of interest related to this review article.