Congenital Malaria in Newborns Selected for Low Birth-Weight, Anemia, and Other Possible Symptoms in Maumere, Indonesia
Article information
Abstract
Congenital malaria is assumed to be a risk factor for infant morbidity and mortality in endemic areas like Maumere, Indonesia. Infected infants are susceptible to its impact such as premature labor, low birth weight, anemia, and other unspecified symptoms. The aim of this study was to investigate the prevalence of congenital malaria and the influence of mother-infant paired parasite densities on the clinical outcome of the newborns at TC Hillers Hospital, Maumere. An analytical cross sectional study was carried out in newborns which showed criteria associated with congenital malaria. A thick and thin blood smear confirmed by nested PCR was performed in both mothers and infants. The association of congenital malaria with the newborn's health status was then assessed. From 112 mother-infant pairs included in this study, 92 were evaluated further. Thirty-nine infants (42.4%) were found to be infected and half of them were asymptomatic. Infected newborns had a 4.7 times higher risk in developing anemia compared to uninfected newborns (95% CI, 1.3-17.1). The hemoglobin level, erythrocyte amount, and hematocrit level were affected by the infants' parasite densities (P<0.05). Focusing on newborns at risk of congenital malaria, the prevalence is almost 3 times higher than in an unselected collective. Low birth weight, anemia, and pre-term birth were the most common features. Anemia seems to be significantly influenced by infant parasite densities but not by maternal parasitemia.
INTRODUCTION
Malaria is an acute systemic illness caused by infection with Plasmodium falciparum, P. vivax, P. malariae, P. ovale, or P. knowlesi, all of which are transmitted to humans by female Anopheles species mosquitoes [1,2]. There are an estimated 135-287 million clinical cases of malaria and 482,000 deaths due to malaria annually in children under 5 years old around the world [3]. In Indonesia, malaria is one of the most common infectious diseases, especially in the Eastern part [4]. Pregnant women and their unborn children are highly susceptible to malaria infection which may cause abortion, premature labor, and still birth [5].
Recently, public transportation and infrastructure have been developed rapidly, causing higher mobility from one area to another, so that the high malaria incidence in Sumba district might have impact on other areas, including Flores (Maumere) which is only 390 km far away. Indonesian Health Surveillance data revealed a significant increase of the infant mortality rate in East Nusa Tenggara, the province of Maumere, compared to non-endemic areas in Central Java. Most causes were perinatal infections and low birth weight (LBW) [6].
Congenital malaria is presumed to be a risk factor for infant morbidity and mortality in Maumere, which is a malaria-endemic area with moderate transmission. In January-June 2013, a preliminary study conducted at TC Hillers Hospital found a prevalence of 14.7% of congenital malaria with species identification of P. falciparum, P. vivax, and a mixture of both in all newborns [7]. To contribute to the understanding of the impact of congenital malaria, the influence of mother-infant paired parasite densities on the clinical outcome of the newborns was studied in TC Hillers Hospital, Maumere, Indonesia.
MATERIALS AND METHODS
Study design and study population
This was an analytical cross sectional study on congenital malaria that included 112 mother-infant pairs and was carried out from December 2012 to December 2013. Samples were taken from a subgroup of newborns with an increased risk of congenital malaria at TC Hillers Hospital, Maumere, and complete blood count was done at a local certified laboratory. Aliquots were then delivered in a container equipped with dry ice to the Faculty of Medicine, University of Brawijaya. Thin and thick blood smear examination was done at the Department of Parasitology, Faculty of Medicine, University of Brawijaya (FMUB), later confirmed by nested PCR examination at the Department of Biomedical, FMUB.
All study participants fulfilled the inclusion criteria, and blood samples were taken aseptically. Clinical symptoms of the newborns were obtained from physical and laboratory examinations.
Sample size
The starting point of this study was when a newborn fulfilled one or more inclusion criteria, and informed consent from the mother or guardian was given. Then, samples from both mothers and newborns were taken. The appropriate sample size was calculated based on comparative statistical analysis formula [8], with estimated congenital malaria proportion of at least 14.7% [7]. The calculated sample size was 64 mother-infant pairs. A total of 112 mother-infant pairs were finally selected according to the following inclusion criteria: 1) premature infants, 2) LBW infants, 3) ill infants aged 1-7 days with symptoms such as anemia, poor feeding, jaundice, seizure, fever, or hepatomegaly. The following exclusion criteria were implemented: 1) asphyxia, 2) multiple congenital anomaly, 3) suspected bacterial sepsis, 4) premature rupture of the membrane ≥ 18 hr, or 5) a traumatic delivery process.
Congenital malaria was defined as an infection resulting from the transmission of parasites from an infected pregnant woman to her fetus. The parasitemia persists after birth and occurs prenatally or during delivery [9]; the asexual forms of malaria parasites (P. falciparum, P. vivax, and mixed infections) present in the peripheral blood smear of the infant in the first 7 days of life, irrespective of clinical symptoms [10,11]. Anemia was defined as a reduction in hemoglobin concentration (Hb), hematocrit (Hct), or the number of red blood cells per cubic millimeter (normal value for at term infant: Hb=14.9-23.7 g/dl, Hct=47-75%; for preterm infant: Hb=17.2-21.2 g/dl, Hct=60-80%) [12,13]. LBW was diagnosed if the baby has body weight less than 2,500 g, irrespective of the gestational age [14]. The preterm infant was defined as birth before 37 weeks of gestation, based on Ballard score [15]. A critical obstetric history was defined as a pregnant woman with previous poor obstetric outcome that can again adversely influence the future pregnancy and labor [16].
Blood smears
Peripheral blood samples of 0.5 ml and 3 ml were taken from infant and mother, respectively. Thick smear and thin smear were prepared according to standard procedures and stained with Giemsa solution. Identification of Plasmodium species using thin smears was performed by 2 independent trained medical analysts. Parasite density was obtained by calculating the number of infected erythrocytes/red blood cells (RBC) per 1,000 RBC and was expressed in percent.
Nested-PCR
DNA samples were extracted from whole blood using of PureLink™ Genomic DNA Kits (Invitrogen, Carlsbad, California, USA) following the manufacturer's instructions. After purification, all samples were stored at -20℃ until further processing for nested PCR. An outer primer pair (rPLU1 and rPLU5) was used to amplify Plasmodium genus sequences; 2 pairs of inner primers for the detection of P. falciparum (rFal1-rFal2) and P. vivax (rVIV1-rVIV2) were used in the second reaction. The PCR was conducted with a Go Tag®Green Master Mix (Promega, Madison, Wisconsin, USA) and 1 µl of the template under the following conditions: 1 µl of each primer (10 µM), 12.5 µl of the PCR master mix, and 9.5 µl of double distilled H2O. The nest 2 reaction was identical except for in the different primers. Sterile distilled water was used as a negative control.
Data collection
Data about the history of pregnancy, physical examination, and anthropometric measurements at birth were obtained. The newborn's health status and the assessment of gestational age were evaluated by a pediatrician. Complete blood examination was conducted at birth.
Statistical analysis
SPSS software version 17 was used for the statistical analyses. A descriptive analysis was conducted to assess the prevalence of malaria and the different Plasmodium species causing congenital malaria infection. After categorization of variables, chi-square test and logistic regression were performed to compare the outcome variables in malaria-positive and negative newborns. Pearson or Spearman's test was performed to measure the correlation between congenital malaria and continuous outcome variables [8]. A significance level of 5% was chosen.
Ethical considerations
This study was approved by the Ethical Committee Medical Research of Medical Faculty University of Brawijaya. Informed consent was obtained from all mothers or legal guardians of the newborns.
RESULTS
Characteristics of samples
From a total of 112 mother-infant pairs who fulfilled the inclusion criteria, 92 were finally selected. The others were excluded due to incomplete or missing data. All included newborns showed a low birth weight.
The baseline characteristics of all newborns are shown in Table 1. Thirty-nine (42.4%) newborns were infected with congenital malaria. Statistical analysis found no significant differences between malaria-positive and negative newborns concerning the gender, birth weight, gestational or maternal age, and the number or history of previous pregnancies (P>0.05).
Clinical presentation of newborns with congenital malaria
The clinical presentations of the infected newborns are shown in Table 2. Nineteen (48.7%) of all newborns with congenital malaria were asymptomatic. There was no statistical difference found in the following symptoms of sepsis, jaundice, prematurity, and asymptomatic LBW infant between infected and non-infected newborns. However, newborns with congenital malaria at TC Hillers Hospital had 4.72 times higher risk in having anemia (95% CI, 1.3-17.1; P=0.018).
Correlation between mother-infant paired parasite densities and outcome of the newborns
To assess the impact of congenital malaria on the outcome of the newborns, birth weight and laboratory findings of each newborn were analyzed. The birth weight of infected newborns (2,016.7±326.3 g) was higher compared to the malaria-negative group (1,986.8±398.9 g). The other parameters such as the hemoglobin level and RBC count were lower in infected infants but neither of the parameters was significantly different from those in non-infected newborns (P>0.05). The mean parasite density of mothers and newborns was 0.34% (95% CI, 0.22-0.46) and 0.96% (95% CI, 0.62-1.31), respectively. The impact of mother-infant paired parasite densities to the outcome of the newborns is shown in Table 3.
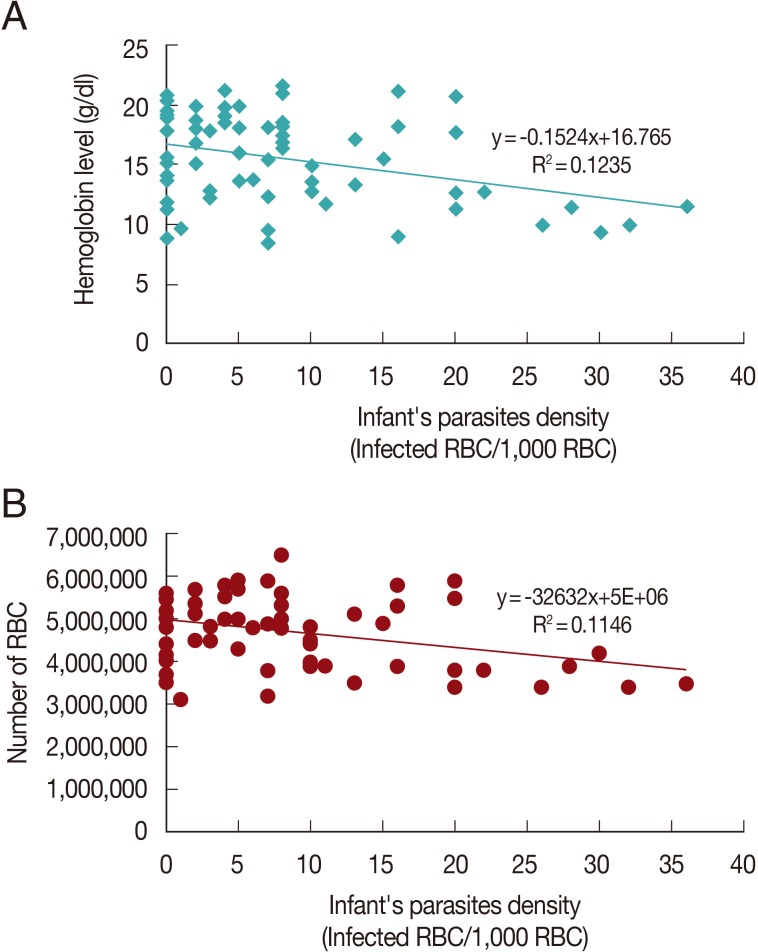
There was no correlation between mother's parasite densities to the outcome of the newborns (P=0.32-0.89) though a tendency for a negative correlation occurred. However, the outcome of the newborns was more affected by their own parasite densities. Significant negative correlations were found for some parameters, such as hemoglobin, RBC count, and hematocrit level (P<0.05). The correlations are shown in Fig. 1. Parasite densities correlated positively with the birth weight but the association was not significant.
DISCUSSION
The present study investigated congenital malaria and its association with anemia, low birth weight, and other variables at TC Hillers Hospital, Maumere, Indonesia. Inclusion criteria were known clinical and laboratory alterations in congenital malaria. The prevalence of congenital malaria in this selected subgroup of newborns was 42.4% whereas the prevalence in all newborns was 14.7% in a recently conducted study at TC Hillers Hospital [7]. If malaria tests are not routinely performed in every mother and newborn in low-to-moderate transmission areas, criteria like LBW, pre-term birth, and anemia should routinely result in tests for malaria. If mother was exposed to malaria during her pregnancy and/or has an origin in high transmission areas, malaria tests should also be performed at birth, especially when the above mentioned criteria are fulfilled.
A previous RCT study, that included 1,030 Mozambican pregnant women, revealed that malaria in pregnancy would have a negative impact on newborn mortality and morbidity [17]. Further studies assessed the influence of congenital malaria on the outcome of the newborns, yet not conclusive [17,18]. One study from 1996-2001 in Cameroonian women showed that placental malaria, as a condition of the occurrence of congenital malaria, reduced the mean infant birth weight by 289 g in primigravida and by 364 g in mothers less than 20 years old [19]. Malaria in pregnancy is thought to reduce birth weight through a combination of systemic and local effects [10,20]. Parasites can either directly cause a disturbance of placental circulation via widespread trophoblast basement thickening, increased fibrinoid necrosis, cytrophoblast prominence or indirectly interfere with the placental functions causing possible pathological lesions [10]. However, many factors-either host or external-can contribute to the incidence of LBW and prematurity, such as maternal age, immune status, gravidity, the use of prophylaxis drugs, nutrition, host or parasite genetics, or the level of transmission [19].
In this study, all included newborns had LBW, as it was one of the inclusion criteria. The differentiation of low (<2,500 g) and very low (<1,500 g) birth weight showed no difference between infected and malaria-negative newborns.
Another parameter analyzed in this study was anemia. This study showed that infected infants had a 4.7 times higher risk in developing anemia (P<0.05). This study also revealed a negative correlation of parasite densities and hemoglobin, erythrocyte count or hematocrit level of the newborns (P<0.05). A previous study conducted in Malawi recorded that the fetal anemia prevalence was about 23%, while in Mozambique up to 93% of newborns were found to have anemia [10]. A study in Southern Malawi found that the mean parasite densities were higher in babies having anemia than in those without it [10].
Congenital parasitic infection can occur at different time points; before birth (in utero) or during delivery. The mechanisms might be by direct penetration through chorionic villi or through premature separation of placenta. The impact of congenital malaria infection manifests as blood flow obstruction, systemic or local inflammatory cytokines and obstacle of transduction signal [21]. The transplacental route is the mandatory mode for transmission of congenital malaria. This route requires parasites to cross and survive or escape of 2 placental barriers, which are the trophoblastic barrier (first barrier) before traversing mesenchymal tissues, surrounding the fetal vessels (second barrier), then finally gaining access to the fetus [9]. This mechanism would explain that maternal parasite density was not associated with fetal parasite density or the outcome of the newborn. Placental barriers might inhibit parasite transmission to the fetus.
Other unspecified symptoms of congenital malaria that might occur were hepatomegaly, jaundice, regurgitation, or poor feeding. The symptoms were found in malaria-positive and negative newborns (P>0.05). One of the co-morbid factors for mortality and morbidity of congenital malaria is the maternal immunity status that is influenced by previous malaria exposure [17]. Most congenital malaria cases were the first or second child. This is similar to placental malaria.
Conclusively, focusing on newborns at risk of congenital malaria, the prevalence is almost 3 times higher than in an unselected collective. The symptoms of congenital malaria varied from asymptomatic LBW newborns to sepsis. Infant parasite density seems to be a determinant factor for the outcome of the newborns, especially for the anemia parameter.
ACKNOWLEDGMENTS
The author would like to thank the staff from the TC Hillers Hospital and to all mothers and newborns who participated in this study. Special thanks to Heni Endrawati S. Si from Department of Parasitology and Tarina Widaningrum S. Si MP from Laboratory of Biomedical, Faculty of Medicine, University of Brawijaya for preparing Giemsa staining and nested-PCR. We also thank Poedji Oetami Amd. K and Mujiatin Amd. K from Central Laboratory of Dr. Saiful Anwar Hospital for their good technical assistance. This study was funded by Faculty of Medicine, University of Brawijaya, Indonesia.
Notes
The authors declare that there is no conflict of interest regarding the publication of this paper.