Immunopathological Changes in the Brain of Immunosuppressed Mice Experimentally Infected with Toxocara canis
Article information
Abstract
Toxocariasis is a soil-transmitted helminthozoonosis due to infection of humans by larvae of Toxocara canis. The disease could produce cognitive and behavioral disturbances especially in children. Meanwhile, in our modern era, the incidence of immunosuppression has been progressively increasing due to increased incidence of malignancy as well as increased use of immunosuppressive agents. The present study aimed at comparing some of the pathological and immunological alterations in the brain of normal and immunosuppressed mice experimentally infected with T. canis. Therefore, 180 Swiss albino mice were divided into 4 groups including normal (control) group, immunocompetent T. canis-infected group, immunosuppressed group (control), and immunosuppressed infected group. Infected mice were subjected to larval counts in the brain, and the brains from all mice were assessed for histopathological changes, astrogliosis, and IL-5 mRNA expression levels in brain tissues. The results showed that under immunosuppression, there were significant increase in brain larval counts, significant enhancement of reactive gliosis, and significant reduction in IL-5 mRNA expression. All these changes were maximal in the chronic stage of infection. In conclusion, the immunopathological alterations in the brains of infected animals were progressive over time, and were exaggerated under the effect of immunosuppression as did the intensity of cerebral infection.
INTRODUCTION
Toxocariasis is a telluric helminthozoonosis due to infection of humans by larvae of Toxocara canis. Infection is acquired by ingestion of embryonated eggs of T. canis, which reach the soil via the feces of infected dogs. After ingestion of eggs, the larvae penetrate the gut to migrate through the liver and other viscera including the central nervous system [1]. Cases of neurological toxocariasis in humans may present as meningoencephalitis with eosinophilic pleocytosis, transverse myelitis with eosinophils in CSF, dementia, cerebral vasculitis, or seizures [2]. Other possible neuropsychological associations include social, cognitive, and behavioral abnormalities especially in children [3]. If detected and treated early, the prognosis of neurological toxocariasis is favorable [2].
In the last 2 decades, the incidence of immunosuppression has surged globally and has strongly influenced medical parasitology. The number of immunosuppressed individuals worldwide continues to increase each year as the human immunodeficiency virus (HIV) pandemic continues to spread unabated in many parts of the world [4,5]. Moreover, in developed nations, the numbers of immunosuppressed individuals continue to increase as a result of medical interventions with aggressive immunosuppressive therapies for immune-mediated disorders, and hematopoietic and solid organ transplants, with approximately 1 million transplants performed annually [5].
The deleterious effects of immunosuppression are documented in certain helminth infections, including strongyloidiasis [6] and experimental trichinellosis [7]. However, few data are available regarding the influence of immunosuppression on cerebral involvement in paratenic hosts infected with T. canis. Therefore, this work aimed to investigate the immunological and pathological brain changes in normal and immunosuppressed mice experimentally infected with T. canis.
MATERIALS AND METHODS
Parasite
Adult T. canis female worms were isolated from the intestine of naturally infected puppies (<3 months). Isolation and embryonation of eggs were performed as follows: female worms were dissected and from the uterus, eggs were isolated and placed into distilled water; then the mixture was centrifuged 2 times for 10 min at 2,000 g in a solution of NaHCl at 1%. After removal of the supernatant, the sediment was washed 2 times in distilled water and placed into the solution of 0.1 N H2SO4 in tissue flasks at 28˚C for 1 month with gentle daily agitation until the end of embryonation which was controlled under the microscope [8].
Drug for immunosuppression
For induction of immunosuppression, a commercial preparation of cyclophosphamide (Endoxan, Baxter, Germany) which contains 1 g/vial was used. The required concentration of the drug was obtained by the appropriate dilution with sterile distilled water. The required dose (20 mg/kg body weight/day for 5 consecutive days) [9] was adjusted to be in a volume not exceeding 0.25 ml. The fine suspension of the drug was injected intraperitoneally within minutes of its preparation.
Animals and experimental design
Laboratory-bred male Swiss albino mice (20-25 g in weight) were used in this study. Mice were housed and infected in accordance with the institutional and national guidelines. A total of 180 mice were divided into 4 groups as follows: group I (30 mice), normal (immunocompetent) non-infected mice as a control group; group II (60 mice), immunocompetent T. canis-infected mice; group III (30 mice), immunosuppressed non-infected mice as a control group; and group IV (60 mice), immunosuppressed T. canis-infected mice. Each animal was infected orally with 1,000 embryonated eggs. Collection of samples was done at week 2, 5, and 12 post-infection (PI). Twenty mice from each of the infected groups and 10 animals from each of the control groups were sacrificed at each time PI, and divided as follows: 10 mice from each of the infected groups for brain larval count, and 10 mice from every 1 of the 4 groups in which the brains were cut longitudinally into 2 equal halves; one for estimation of IL-5 mRNA expression levels in the brain homogenates, and the other for pathological, histochemical, and immunohistochemical study. Only 5 mice were randomly selected for cytokine estimation.
Total larval counts in the brain
Infected animals (10 mice at each time PI) were euthanized, and the whole brain from each animal was removed in a Petri dish. The 2 halves of the cerebrum and the cerebellum were separated and each was compressed between microscope slides and examined under the low power of a light microscope. The larvae were counted directly and their motility was observed.
IL-5 mRNA expression by semi-quantitative real-time PCR
Brain samples (5 randomly selected from the infected groups and control groups at week 2, 5, and 12 PI) were processed for total RNA extraction (MagNA Pure compact Nucleic Acid isolation kit I, Roche Diagnostics, GmbH, Mannheim, Germany) according to the manufacturer’s recommendations. First strand cDNA was synthesized from total RNA using Transcriptor First Strand cDNA Synthesis Kit (Roche, Germany) according to the manufacturer’s instructions.
IL-5 mRNA transcripts were quantified, relative to the house-keeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the Roche LightCycler® FastStart DNA MastePLUSSYBR Green I kits (Roche Diagnostics) following manufacturer’s instructions. The primer sequences for IL-5 and GAPDH used in the study were as follows: IL-5 (Sense 5-GGTGTATTCGTTGGCCTCCTG-3), IL-5 (antisense 5-GCCTAGCAATAGAGATCCTTG-3), GAPDH (sense 5´GAAGGTGAAGGTCGGAGTC-3´), GAPDH (antisense 5´-GAAGATGGTGATGGGATTTC-3´). The optimal conditions for PCR amplification were: an initial incubation at 95˚C for 30 sec, 45 cycles of denaturation at 95˚C, annealing at 55˚C (10 sec), and extension at 72˚C (13 sec). The values of the target gene under investigation and the value of the housekeeping gene (GAPDH) were calculated for each sample using a standard curve. The standard curve for each target gene and the house keeping gene was made by 3-fold serial dilutions of a single cDNA sample from a non-infected control mouse, and the obtained crossing point (CP) values were plotted against an arbitrary log concentration. A ratio between the calculated values of the target gene and housekeeping gene was obtained, which indicates the amount of target normalized to the level of an endogenous reference gene within each unknown sample. The final results were automatically calculated from the CP values of the target and the reference genes by LightCycler® 480 Relative Quantification Software (Roche Applied Science, Indianapolis, Indiana, USA).
Histopathological study
All the studied specimens were fixed in 10% formalin and subsequently embedded in paraffin. The sections were stained with hematoxylin and eosin (H&E) and periodic acid Schiff (PAS). The stained sections from the infected animals were examined in comparison with their controls.
Immunohistochemistry was done for demonstration of activated glial cells using antibody against glial fibrillary acidic protein (GFAP). Immunohistochemical staining was performed on 3-5 μm sections from randomly selected 10 paraffin blocks from the infected group and 5 blocks from the control group at week 2, 5, and 12 PI, using the Ultra Vision Detection System (Anti-Polyvalent, HRP/DAB ‘‘Ready-to-Use’’, Cat. #TP-015-HD, Lab Vision, USA). The immunostaining was conducted according to the manufacturer’s protocol. Briefly, an overnight incubation of the sections with antibodies against GFAP (Ab-4 rabbit polyclonal antibody, Cat. #RB-087-R7 ‘‘Ready-to-Use’’, Lab Vision) was done at room temperature in a humidity chamber. The sections were then washed with PBS and incubated with biotinylated goat anti-polyvalent for 10 min at room temperature followed by washing with PBS, then incubated with streptavidin peroxidase solution for 10 min at room temperature, then rinsed with PBS. The reaction products were visualized using 3-3´-diamino-benzidine-tetra-hydrochloride (DAB), and the sections were then counterstained with Mayer’s hematoxylin, dehydrated in alcohol and mounted in Di-n-butyl-phthalate-polystyrene-xylene (DPX). In order to confirm the results of the staining, sections from normal mouse lung and brain were used as positive controls for GFAP. In addition, for negative controls, omission of the primary antibodies was done, and instead, normal rabbit serum was applied. Cells showing a distinct brownish cytoplasmic reaction to GFAP were considered positive.
Quantification of GFAP immunoreactivity
For the evaluation of GFAP staining, a semi-quantitative scoring was applied for both cell number (proportional score) and staining intensity (intensity score). The number of positive cells was given as 0=0%, 1=<5%, 2=5-25%, 3=26-75%, and 4=76-100% of cells showing cytoplasmic positivity. The intensity of staining was graded on a scale consisting of 0 to 3 (0=negative, 1=weak, 2=moderate, and 3=strong). The total score was calculated by the summation of both proportional score and intensity score. This sum score was then divided into 4 grades as follows: grade 0=0, grade 1=1-2, grade 2=3-4, and grade 3=5-7 [10,11]
Statistical analysis
Data were presented as mean±SD. Analysis of variance (ANOVA) test was used for comparison among different groups for quantitative data. Fisher’s exact test was used for evaluating GFAP expression. Differences were considered significant when P-value was <0.05. The statistical analyses were processed according to the conventional procedures using Statistical Package of Social Sciences (SPSS Inc., Chicago, Illinois, USA) software for windows, version 10.0.
RESULTS
Brain larval count
Table 1 shows larval counts in the brain of infected immunocompetent and immunosuppressed animals. There was a significant increase of larval recovery as the infection progressed. Moreover, there was a significant increase of larval recovery in the immunosuppressed group in comparison with the immunocompetent group at all time points PI. Focal aggregation of larvae within the brain was observed, and the larvae were coiled or occasionally moving slowly. These qualitative characteristics were similar in both groups of mice.
IL-5 mRNA expression in brain homogenates
There was a significant increase (P<0.05) of levels of IL-5 mRNA expression in immunocompetent infected mice compared to normal controls (Fig. 1). The rise in IL-5 mRNA levels started at week 5 PI and continued to week 12 PI. Meanwhile, there was a significant decline of levels of IL-5 mRNA expression in the immunosuppressed infected group compared with the immunocompetent infected group from week 2 PI onwards.

IL-5 mRNA expression in the brain tissues at different time points post-infection (PI). Vertical bars represent the mean (±SD) of these results for each group (n=5). (A) Significant increase (P<0.05) was noted in levels of IL-5 mRNA expression in immunocompetent infected group at week 5 and 12 PI as compared to normal controls. (B) Significant difference (P<0.05) was noted in levels of IL-5 mRNA expression in both infected groups at all durations PI.
Histopathological and histochemical findings
Histopathological examination revealed the presence of numerous sections of T. canis larvae scattered in the parenchyma of the brains of infected group (Fig. 2A), especially near the choroid plexus and corpus callosum, with fewer larvae detected in the cerebellum. No visible inflammatory reaction was observed around the migrating larvae. Larvae were more abundant in brain sections from the immunosuppressed mice, and, similarly, no apparent inflammatory reaction was observed in the brain sections of the immunosuppressed mice.

Photomicrographs of brain sections showing (A) Numerous tangential and cross-sectional profiles of Toxocara larvae (arrows) deposited within the cerebral tissue. No apparent inflammation was noticed (H&E, ×400). (B) PAS-positive material deposited in the wall of blood vessels (arrows) (PAS, ×400).
By using PAS stain, we observed the deposition of PAS-positive material in the walls of blood vessels. The PAS-positive material took the linear pattern with variable thickness (Fig. 2B). Intense deposition of homogenous PAS-positive material was also detected in the larvae in the brain. Most of the sections showed patchy deposition of PAS-positive material in the stroma. The changes were similar in both immunocompetent and immunosuppressed groups.
GFAP immunoreactivity
Immunohistochemical assessment by GFAP immunoreactivity showed a significant increase in GFAP expression by activated astrocytes in the infected groups, localized in the cerebral parenchyma particularly near the choroid plexus and corpus callosum (Table 2). Weak GFAP expression was detected in the age-matched control groups. Increased GFAP expression was detected as early as week 2 PI in immunocompetent infected group (Fig. 3A), and it increased significantly throughout the course of infection (Fig. 3C). Furthermore, the immunosuppressed group showed a significantly higher GFAP immunoreactivity by activated astrocytes as shown by the increased intensity of staining and increased number of astrocytes. The increase in GFAP expression was also progressive over time (Fig. 3B, D).
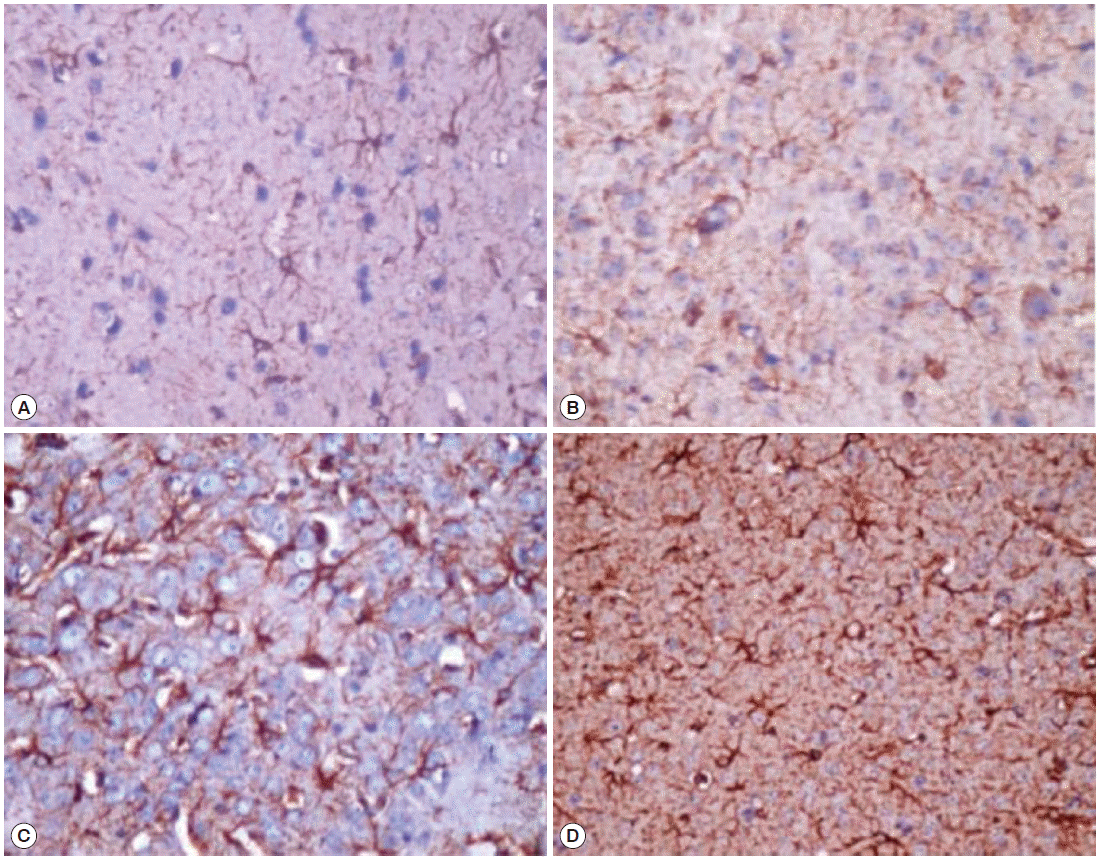
GFAP staining of activated astrocytes. (A) Immunocompetent infected group at week 2 post-infection (PI) showing grade 1 immunoreactivity. (B) Immunosuppressed infected group at week 2 PI showing grade 2 immunoreactivity. (C) Immunocompetent infected group at week 12 PI showing grade 3 immunoreactivity. (D) Immunosuppressed infected group at week 12 PI showing grade 3 immunoreactivity (immunoperoxidase stain, ×400).
DISCUSSION
Toxocariasis is one of the most commonly reported zoonotic helminth infections in the world [1]. There is ample evidence that T. canis has the potential to alter the behavior of the host due to the neurotrophic nature of the larvae [12]. Therefore, experimental cerebral toxocariasis can provide insights into host–parasite interactions, which could be relevant to human infections [12]. Meanwhile, nowadays, there is an increased incidence of immunosuppression due to various causes such as cancer, and immunosuppressive therapy for neoplasia, collagen diseases, and organ transplantation [5]. Despite the immense impact of helminthiases on the human health and their widespread nature, the study of parasitic helminth infections, including toxocariasis, has relatively received little attention in the immunosuppressed hosts.
In the current study, there was progressive accumulation of larvae in the brain over time in both infected groups and a statistically significant increase in the larval burden in the brain of immunosuppressed mice relative to the immunocompetent mice. These results were in agreement with those of Abo El-Asaad et al. [9] who reported significant increase in the brain larval count in immunosuppressed animals. Accumulation of more larvae in the immunosuppressed group may be due to arrival of a large number of migrating larvae to the brain because of deficiency of inflammatory reactions under the effect of cyclophosphamide. el Ridi et al. [13] and Mariotti et al. [14] demonstrated that cyclophosphamide has a suppressor effect on T cells and inflammatory reaction. Therefore, the inhibition of the inflammatory reaction in the liver and other organs has presumably led to migration of more larvae from these organs to the brain.
Likewise, experimental hymenolepiasis nana showed significant increase in infection intensity, significant reduction in intestinal mast cell count, and dissemination of larvae to the liver under the effect of immunosuppression with cyclophosphamide [15]. Additionally, El Kowrany et al. [16] reported that there was increased number of Heterophyes heterophyes adult parasites in the intestine of mice under the effect of immunosuppression with cyclophosphamide. Furthermore, Budovsky et al. [17] found that there was heavy parasitemia after administration of cyclophosphamide in rats infected with Trypanosoma lewisi.
Histopathological examination of brain sections showed no leukocytic infiltration or evident pathological changes despite the presence of Toxocara larvae in the brain. The reason for the absence of inflammatory cell infiltration in the injured Toxocara-infected brain might be that T. canis larvae mimic host tissue antigenic components and escape immune recognition, or perhaps some mechanisms in the nervous tissues operate to diminish inflammation in order to protect themselves from severe injuries caused by inflammation. Our findings were in agreement with Liao et al. [18] and Othman et al. [19] who reported the same observations. From a qualitative point of view, the immunosuppressed infected group showed the same findings but the difference was the increased number of larvae in the brain.
In our study, the brain sections were stained with PAS which stains the neutral and alkaline mucopolysaccarides in the tissues and also stains the basement membranes. We found that PAS-positive material was deposited in blood vessel walls and in adjacent stroma. Similarly, Eid [20] found that experimental Schistosoma infection had a similar picture in the brain with PAS-positive material detected in blood vessel walls and in the adjacent stroma which was explained to be due to immune complex mediated reaction.
Astrocytes are supportive structural elements of the nervous system. They play active roles in normal brain physiology and in certain pathological conditions. They respond to brain injury by releasing cytokines, increasing levels of some specific proteins, increasing their volume and forming a dense meshwork of processes–a process called gliosis [21]. Notably, GFAP is a glial biomarker used to help delineate pathophysiological mechanisms and monitor neurological outcome of brain injury resulting from either physical, chemical or biological insults. Reactive astrocytes with increased expression of GFAP are commonly found in cerebral infarction and many areas of brain damage [22].
Although ordinary histological staining showed no leukocytic infiltration or apparent pathological changes in areas near the choroid plexus, the site of T. canis invasion, there was evidence of ongoing cerebral injury, as presented with the observed astrogliosis with enhanced expression of GFAP, extending around injured areas. The levels of expression appeared to be correlated with the number of larvae migrating into the brain over time, increasing progressively from week 5 to 12 PI. These changes may be a response to help protect the brain from damage in experimental neurotoxocariasis. Activated astrocytes can extend far from actual site of damage and help reestablish an intact blood–brain barrier [22]. In the immunosuppressed infected group, it was found that GFAP expression was enhanced in relation to that in immunocompetent infected group, and additionally, this increase was progressive over time. This could be explained by retention of more larvae in the brain. Notably, previous research have demonstrated that GFAP expression is enhanced in the brains of Toxocara-infected mice [18,19].
IL-5 plays a unique and specific role in control of eosinophil production and differentiation (eosinophil differentiation factor; EDF), and it may also be able to activate basophils. Moreover, IL-5 is closely associated with eosinophilia in helminth infection [23]. Thus, Yamaguchi et al. [24] demonstrated the enhanced expression of IL-5 mRNA in splenic cells from T. canis-infected mice, confirming the major role of IL-5 in induction of peripheral eosinophilia; the latter was further suppressed by administration of anti-IL-5 monoclonal antibodies.
In the present work, there was a significant increase in expression of IL-5 mRNA over time with infection. Similarly, Hamilton et al. [25] have demonstrated that IL-5 mRNA expression was enhanced in the brains of Toxocara-infected mice. Furthermore, they reported that this increased expression was detected as early as day 3 PI and persisted up to day 97 PI. In this work, IL-5 mRNA levels in immunosuppressed mice were significantly lower than those in immunocompetent mice. This may be due to the effect of cyclophosphamide which has a suppressor effect on T lymphocytes and the inflammatory reaction. Despite the absence of eosinophilic infiltration in the brains of infected mice, these data may be more relevant to other paratenic hosts, such as humans and rats. In these hosts, T. canis induces florid eosinophil-rich granulomatous inflammatory reaction ending in a scar formation [12]. The inflammatory response may destroy the larvae or at least limit their mobility within the nervous tissue.
Although cases of neurotoxocariasis were reported in humans, Toxocara infection is largely neglected as a differential diagnosis of neurological disorders. If toxocariasis is neglected or ignored as a differential of these abnormalities, it may be easily overlooked for years. Therefore, high priority should be given to further studies of Toxocara infection in the community, and neurotoxocariasis should receive more attention and a large scale investigation in humans. As the diagnosis of toxocariasis is not always feasible, and as the treatment at present is far from ideal, largely due to lack of drugs with well-documented efficacy against Toxocara larvae, individual and community prophylactic measures are advisable, especially in those individuals suffering from impaired immunity due to a variety of causes.
In conclusion, experimental T. canis infection induced significant immunological and pathological changes in the brains of infected animals. Under the influence of immunosuppression, these changes were more pronounced, while the immune response was blunted, evidenced by suppressed levels of IL-5 mRNA. Also, it was found that these changes were more evident in the chronic phase of infection. Further studies are warranted as regards the course of toxocariasis in various conditions of immunosuppression. Finally, more studies should be done to establish the immunopathogenic mechanisms of neurotoxocariasis which may open avenues for future therapeutic options.
Notes
We have no conflict of interest related to this study.

