Seroprevalence and Potential Risk Factors Associated with Neospora spp. Infection among Asymptomatic Horses in Jordan
Article information
Abstract
This study aimed to determine the seroprevalence and to identify risk factors associated with Neospora spp. infection in horses in Jordan. Management related data were collected from each farm and individual horses. Sera from 227 horses from 5 of 6 climatic regions in Jordan were analyzed for the presence of antibodies to Neospora spp. by ELISA kit. The study was performed during spring of 2010. The association between seropositivity and risk factors was analyzed. A total of 7 (3%) of 227 sera had antibodies for Neospora spp. There was a significant regional difference (P=0.018) between the 5 climatic regions. Positive cases were located in Amman and Irbid, while the other regions (Zarqa, Jordan Valley, and Wadi Mousa) had zero prevalence. The use of anthelmintics at least once a year resulted in a significant reduction of the seroprevalence to Neospora spp. (1.6% vs 9.8%). However, this might be a phenomenon by chance and a better hygiene since owners can invest in anthelmintics. Other risk factors such as age, gender, breed, usage, body condition score, grazing, presence of other animals mixed with the horses in the same property, and a history of previous diseases were not significantly associated with the seroprevalence to Neospora spp. infection. This is the first study to report on the presence of Neospora seropositive horses in Jordan. Further studies are warranted to better understand the role of certain risk factors in the transmission of Neospora spp. among horse population and to determine which Neospora spp. are responsible for the infection.
INTRODUCTION
Equine neosporosis is caused by an obligatory intracellular apicomplexan protozoan parasite, Neospora caninum. N. caninum belongs to the family Sarcocystidae and closely resembles Toxoplasma gondii [1]. Neospora hughesi is a second species of Neospora described in the horse [2]. The parasite infects a broad range of animals including cattle, sheep, goats, deer, horses, and dogs. Although N. caninum is an important cause of abortion in cows, as well as various congenital abnormalities in dogs, little is known regarding its pathogenicity and transmission in horses [3]. Exposure to Neospora spp. in horses is not uncommon, clinical disease associated with natural infections in adult horses, however, have been reported in only few cases. Moreover, it is uncertain whether N. caninum, N. hughesi, or both are responsible for the disease in positive cases since both species cross-react serologically [4]. In horses, infection with Neospora spp. has been associated with neurological disorders, neonatal diseases, and abortion [5]. Various laboratory techniques have been used to diagnose N. caninum-infected animals. Immunohistochemistry is used to demonstrate N. caninum in the placenta or fetal tissues [6]. Serology tests such as indirect fluorescent antibody test (IFAT), immunoblotting (IB), direct agglutination tests, and a wide variety of ELISAs are used to detect specific antibodies in sera of infected animals. In addition, DNA biotechnology can be used to distinguished N. hughesi from N. caninum [7].
According to the Jordan Ministry of Agriculture Yearly Statistical Data (2007), the total population of horses is 2,182 heads. In Jordan, the prevalence and risk factors associated with N. caninum infection has been reported in dairy herds [8]. Information regarding the prevalence of Neospora spp. in horses is lacking, therefore, the purpose of the study reported here was to determine the seroprevalence of Neospora spp. in horses and to identify potential risk factors for neosporosis in horses in Jordan.
MATERIALS AND METHODS
Study animals and design
Total 227 clinically normal horses were enrolled in the study. The horse farms were selected randomly using the records of the Jordanian Ministry of Agriculture. Horses from each farm were selected randomly using a table of random digits. Horses sampled for the study were from 5 of 6 climatic regions in Jordan; Amman, Irbid, Zarqa, Jordan Valley, and Wadi Mousa (Fig. 1). The 6th region was not sampled due to the lack of horse population. About 40-50 horses were sampled from each region. The study was performed during spring of 2010. Each farmer was interviewed to gather information about each horse, farm characteristics, and herd management. Table 1 shows a list of specific data that was collected on each horse and the farm included in the study.
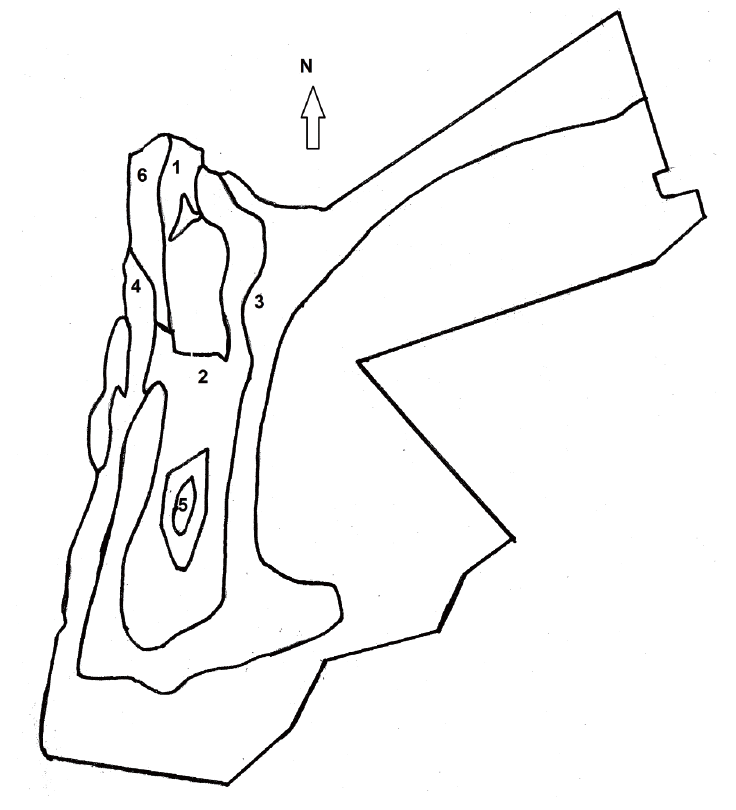
Map of Jordan divided into 6 climate regions according to the model of Koppen (anon., 1984). It also indicates the location of 5 sites sampled in this study. 1. Warm temperature rainy (Irbid), 2. Cool steppe (Amman), 3. Cool desert (Zarqa), 4. Warm desert (Jordan Valley), 5. Cool temperate rainy (Wadi Mousa), and 6. Warm steppe (not sampled).
Blood samples
Whole blood (5 to 10 ml) was collected from the jugular vein of each horse using vacuum plain tubes (Ayset tube®, Adana, Turkey) and transported on ice to the laboratory within 2hr. Blood was centrifuged at 5,000 g for 10 min, and sera were harvested and stored in microtubes at -20˚C until analyzed.
Laboratory analysis
For determination of Neospora spp. seroprevalence, antibodies in sera were detected by an indirect ELISA kit (The HerdChek Anti-Neosporai caninum Antibody Test Kit®, IDEXX Laboratories, Westbrook, Maine, USA) according to the manufacturer’s instructions. The test has a known sensitivity and specificity of 98.6% and 98.9%, respectively. The resulted prevalence was adjusted to the test sensitivity and specificity using the formula published previously [9].
Statistical analysis
The seroprevalence and risk factor analysis of Neospora spp. infection were determined using the chi square tests of association [10]. Variables with a P-value of <0.05 were considered statistically significant.
RESULTS
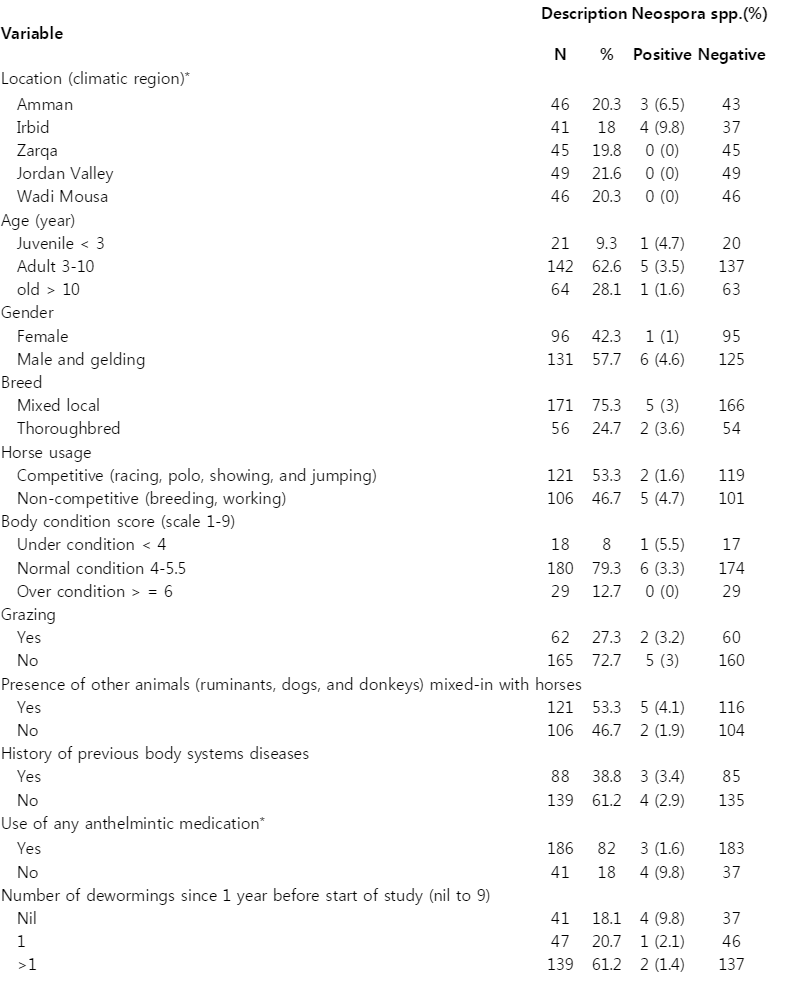
Table 1 shows the distribution of Neospora spp. positive and negative horses (n=227) from Jordan with various risk factors that might be associated with the seropositivity to equine neosporosis. The overall seroprevalence of Neospora spp. was 3% (7/227 horses). There was a significant regional difference (P=0.018) with the positive cases belonging to Amman and Irbid. The following risk factors were not significantly (P>0.05) associated with seroprevalence of Neospora spp.: horse age, gender, breed, usage, body condition score on a scale of 1-9 (a score of 1 represents emaciation whereas a score of 9 is a profound obesity), grazing, presence of other animals mixed with the horses in the same property, and a history of previous diseases such as gastrointestinal (colic and diarrhea), respiratory (fever, coughing, naso-ocular discharge), integumentary (dermatitis), and musculoskeletal diseases (lameness) since 1 year before the commencement of the study. Of 227 horses, 186 (82%) received anthelmintic medication at least once or more a year. Ivermectin and/or piprazine were the most commonly used anthelmintics. Greater than or equal to 1 deworming per year was significantly (P=0.0017) associated with a reduction in the seroprevalence of Neospora spp. (1.6% vs 9.8%). This finding was supported by an effect of any drench reducing the seroprevalence of Neospora spp. (P=0.0063). Of 186 horses drenched, only 3 cases (1.6%) of Neospora infection were identified. In contrast, the other 4 cases of Neospora infection were identified from 41 horses (9.8%) that had not been drenched for worms. Testing the association of specific anthelmintic drugs with the seroprevalence of Neospora spp. was not possible due to the low seroprevalence of Neospora.
DISCUSSION
Neosporosis in horses has been reported from different parts of the world. To the authors’ knowledge, this is the first study in Jordan to determine the prevalence of antibodies to Neospora spp. and to evaluate risk factors associated with the occurrence of neosporosis in horses. Unfortunately, we were unable to determine which Neospora spp. were infected the seropositive horses since both species cross-react serologically [4]. The results of this study indicated that horses in Jordan are exposed to Neospora spp. with an overall seroprevalence of 3%. This percentage is considerably lower than the seroprevalence of Neospora infection that have been reported in horses from the United States [11] and Czech Republic [12]. In the Middle East, seroprevalence of Neospora spp. was reported from Israel [13] and Saudi Arabia [14]. The seroprevalence of Neospora spp. infection in horses is considerably different among and within countries. These variations might be due to differences in the serological tests used in each study, limitations of the testing methods, and non-standardized controls, and cut-off values applied [15]. In addition, the study design, criteria for sample collection, different levels of exposure to the various risk, and protective factors for infection or disease might influence the seroprevalence of Neospora spp. which make the comparison between results from different studies difficult [16].
Determining various risk factors and understanding their role in disease transmission and epidemiology is critical for the development and implementation of proper measures to control equine neosporosis. The seroprevalence of Neospora spp. was higher in Amman and Irbid compared to other climatic regions of the country. It was noticed that these 2 regions have the highest density of horse population in Jordan. Significant differences in the seroprevalence of Neospora spp. among horse groups from various geographical regions were reported from USA [15]. However, no significant difference was found in the seropositivity rates from 4 districts in Niğde province of Turkey [17].
The present study showed a non-significant decrease in the seroprevalence of Neospora spp. with increased horse age. This result is contrary to the previous report from Israel [13] which found a significantly higher seroprevalence to Neospora spp. as horses get older than 10 years of age (because of an increased likelihood of exposure through horizontal transmission over time). In a Turkish study, a non-significant increase of seroprevalence to Neospora spp. (22.2% and 27.2%) was found between 2 age groups; 1-10 and 11-20 years old, respectively [17]. The absence of significant effect for the gender on the seroprevalence of Neospora spp. in this study is in agreement with findings of Villalobos et al. [18]. The horse breed was also not significantly associated with the seroprevalence to Neospora spp. which agrees with the study of Kligler et al. [13] in Israel. The presence of other animals mixed-in with horses in the same property was also not significantly associated with seroprevalence to Neospora spp. In cattle, it is generally accepted that the presence of farm dogs, the definitive host for N. caninum, increases the chance of N. caninum infection [19]. The present study showed that the use of anthelmintic medication at least once a year resulted in a significant reduction in the seroprevalence of Neospora spp. However, this might be a phenomenon by chance and a better hygiene since owners can invest in anthelmintics. In cattle, various antimicrobial agents have been tested against N. caninum in vitro but there is still no safe and effective chemotherapy that clears infection completely [20]. However, in vitro and in vivo experimental studies have shown a promising effect for toltrazuril and ponazuril on tachyzoites of N. caninum in calves [21]. Further studies are necessary to determine which species, N. caninum or N. hughesi, infect the horses. Furthermore, detailed studies to better understand various risk factors that affect the transmission of the parasite among horses are warranted.