Low Fetal Weight is Directly Caused by Sequestration of Parasites and Indirectly by IL-17 and IL-10 Imbalance in the Placenta of Pregnant Mice with Malaria
Article information
Abstract
The sequestration of infected erythrocytes in the placenta can activate the syncytiotrophoblast to release cytokines that affect the micro-environment and influence the delivery of nutrients and oxygen to fetus. The high level of IL-10 has been reported in the intervillous space and could prevent the pathological effects. There is still no data of Th17 involvement in the pathogenesis of placental malaria. This study was conducted to reveal the influence of placental IL-17 and IL-10 levels on fetal weights in malaria placenta. Seventeen pregnant BALB/C mice were divided into control (8 pregnant mice) and treatment group (9 pregnant mice infected by Plasmodium berghei). Placental specimens stained with hematoxylin and eosin were examined to determine the level of cytoadherence by counting the infected erythrocytes in the intervillous space of placenta. Levels of IL-17 and IL-10 in the placenta were measured using ELISA. All fetuses were weighed by analytical balance. Statistical analysis using Structural Equation Modeling showed that cytoadherence caused an increased level of placental IL-17 and a decreased level of placental IL-10. Cytoadherence also caused low fetal weight. The increased level of placental IL-17 caused low fetal weight, and interestingly low fetal weight was caused by a decrease of placental IL-10. It can be concluded that low fetal weight in placental malaria is directly caused by sequestration of the parasites and indirectly by the local imbalance of IL-17 and IL-10 levels.
INTRODUCTION
Malaria in pregnant women can cause severe clinical manifestations both in the mother and fetus. In areas where malaria is endemic, around 100,000 infant deaths each year could be due to low birth weight (LBW) caused by malaria during pregnancy [1]. Indonesia is a country that is still at risk of malaria, especially in well-known endemic areas, such as the Eastern region of Indonesia. The number of malaria cases in 2010 was 142,238 and in 2011 was 129,550, while pregnant women suffering from malaria reached 3,896 cases [2].
The presence of malaria parasites in pregnant women would lead to accumulation or sequestration of infected erythrocytes in the placenta intervillous space through chondroitin sulfate A (CSA) receptors and lead to placental malaria [3]. This process induces the parasites to release bioactive molecules such as glycosylphosphatidylinositol (GPI) that stimulate maternal mononuclear cells [4,5] and fetal syncytiotrophoblasts to produce inflammatory cytokines and chemokines [6,7]. These cytokines and chemokines further recruit, retain, and activate mononuclear cells in the placenta [8].
Although it has been known that infected erythrocytes and accumulation of cytokines and chemokines in the placenta may result in adverse pregnancy as intrauterine growth restriction (IUGR) of the fetus, how certain immunological mechanisms cause damage to the fetus and placenta remains a question until present. As intervillous space is a main compartment for the delivery of nutrients and oxygen and help the development of the fetus, an adverse outcome of placental malaria might be triggered by chemokines and cytokines leading to impaired materno-fetal exchange and damage to the placenta [3].
In normal pregnancy tumor growth factor (TGF-β), IL-6, and IL-1 induce Th17 to produce IL-17 [9]. IL-17 plays important roles for induction of inflammation [10] which is necessary for successful implantation [11]. However, after the implantation process is completed, the dominance of Th1/Th17 response shifts to Th2 responses, marked by release of IL-4, IL-9, and IL-10. This phenomenon is regulated by regulatory T (Treg) cells [12], so that the growth of decidual and trophoblast cells in the placenta can be assured to take place normally.
Infected placentas showed an increase in inflammatory molecule levels, such as TNF, IL-8, and IL-6 for parasites clearance [13,14]. While IL-6 induces Th17 to produce IL-17, these can lead to an inflammatory response that would lead to pregnancy failure [12]. The high Th1 cytokines such as TNF-α and IL-6 also stimulate lymphocytes to produce Th2 cytokines such as IL-10, which has the effect of pressing Th1 dominance as immunoregulator [14]. Based on the description above, this study aimed to prove the role IL-17 and IL-10 of the placenta in the pathophysiology of pregnancy disorders due to malaria infection.
MATERIALS AND METHODS
Research design and sample
This study had been registered and approved as stated by the Health Research Ethics Committee of the Faculty of Medicine, Universitas Brawijaya in statement of ethical clearance no. 104 dated 7 March 2014. This research was conducted at the Laboratory of Parasitology and Biomedical Laboratory, Faculty of Medicine, Universitas Brawijaya Malang, Indonesia. The research involved 50 of 13-15 weeks old, 20-30 g in weight, and health female BALB/c mice, which had been synchronized their estrus cycles by using the phenomenon of Leeboot, Pheromone, and Whitten effect [15]. The female mice then were simultaneously mated in pair within 1 night and then were randomly devided into 2 groups. Female mice from the first group were intraperitonelly infected by Plasmodium berghei on the 9th day of post mating and used as the treatment group, whereas the other group that was not infected was used as the control group. Those 2 groups of mice were then evaluated daily, especially on their physical condition, body weights, and pregnancy symptoms. All mice were scariscarified on the18th day post mating. After the mice were anesthesized using chloroform, blood was aspirated intracardially and the abdominal walls were opened to identify their pregnancy status. The fetus from pregnant mice of both groups were weighed invidually using analytical Mettler AE 50 (Toledo, Ohio, USA). The placenta was isolated to prepare histopathological slides with hematoxylin and eosin (H&E) and to check true for cytokine levels.
The levels of placental tissue IL-17 were measured using Quantikine ELISA (R&D Systems, Abingdon, UK), while the placental levels of IL-10 were measured using mouse IL-10 ELISA kit from Abcam (Cambirdge, Massachusetts, USA).
Synchronization of estrus and mating
Fifty female mice were divided into 5 cages and separated from male mice for 2-3 weeks. The female mice were in a condition of un-estrus state (Leeboot effect). They were then exposed to odors of males in order to restart the estrus cycle (Pheromone effect). The female mice were simultaneously in estrus condition for about 72 hr after being exposed to male odor (Whitten effect). Finally they were simultaneously mated in pair (1:1) within 1 night [15].
Plasmodium berghei ANKA strain inoculation
On the 9th day post mating, mice from the study group were inoculated with as much as 1×106 of Plasmodium berghei ANKA strain (first passage) per ml of blood intraperitoneally.
Isolation of placenta and fetus
Isolation of placenta and fetus was done on the 18th day post mating. The suspected pregnant mice (physically) were scarified under anesthesia with chloroform, and surgery was performed by opening the abdominal wall to take the uterus. The fetus were weighed individually, and the placentas were divided into 2 parts; a part isolated then stored in -80˚C for analysis of placental cytokines and the other part fixed with 10% formaldehyde for histopathological studies after H&E staining.
Examination and measurement of cytoadherence
Examination of cytoadherence was performed on the histopathological slides stained with H-E at the Laboratory of Pathological Anatomy dr. Sutomo Hospital, Faculty of Medicine, Universitas Airlangga Surabaya, Indonesia.
The slides of placental tissue which had been stained with H-E were then examined by 2 independent examiners using a light microscope under 1,000×magnifications. The levels of cytoadherence were determined by counting the number of parasitized red blood cells (RBCs) among 1,000 RBCs in the intervillous space of placenta by using 2 hand counters.
Isolation of placental tissues for cytokine measurement
Placental tissues of each mouse were homogenized with 0.1 M Tris-buffered saline (pH 7.4) containing 0.5% Triton X-100 and 1 tablet of Complete Mini protease inhibitor cocktail tablets/10 ml (Roche Diagnostics, Indianapolis, Indianapolis, USA). Afterwards, they were centrifuged at 15,000 rpm for 30 min. The supernatant was collected, and protein concentration was measured. They were then kept at -80˚C until used for assay [16,17].
Examination of IL-17 and IL-10 levels in the placenta
The levels of IL-17 and IL-10 in placentas were determined by ELISA. Fifty μl of assay diluent RD1-38 was added to each well that had been coated with the primary antibody, and samples were added as much as 50 μl per well. Samples were combined by mixing the plate frame for 1 min then covered with adhesive strip and incubated for 2 hr at room temperature. Each sample was aspirated and then washed for 4-5 times. Samples were washed by filling each well with wash buffer (400 μl) by spraying it with a dispenser. After the last wash, the remaining wash buffer was removed by tapping the plate on a clean paper towel. Then, 100 μl of secondary antibody that had been conjugated with biotin was added to each well. The plate was covered with new adhesive strip and incubated for 2 hr at room temperature. The washing process was repeated 3 times. After that, 100 μl of substrate solution were added to each well and incubated for 30 min at room temperature and protected from light. Finally, 100 μl of stop solution was added to each well. Within 30 min, the plate was read under a ELISA reader at a wavelength of 450 nm.
Data analysis
The data were analyzed by using Structural Equation Modeling (SEM) method with true tool of Smart Partial Least Square (PLS) software. The purpose of this analysis was to build and test the statistical model in the form of causal models.
RESULTS
On the 18th day post mating, among the total 50 female mice, there were only 17 mice approved to be pregnant and were eligible for this study, those were 9 mice from the infected/treatment group and 8 mice from the un-infected/control group. It means that the pregnancy rate after synchronization of estrus and mating in pair within 1 night in this study was only 34%.
Levels of cytoadherence
The mean cytoadherence on the 18th day post mating or 9th day post inoculation was 33.6±17.2% however, the data were not homogenous. The cytoadherence of erythrocytes infected by P. berghei in the placenta is shown in Fig. 1.

Cytoadherence of Plasmodium berghei infected erythrocytes in the placenta. (A) Placenta of control group/non-infected pregnant mice: no sequestration. (B) Placenta of treatment group/infected pregnant mice. Arrows indicate infected erythrocytes undergoing sequestration in the placental intervillous space (examined by Olympus Microscope under 1,000 × magnification).
Levels of placenta IL-17 and IL-10
Levels of placenta IL-17 and IL-10 in the treatment and control groups were visualized on the box-plot diagram (Figs. 2, 3). The ratio of IL-17 to IL-10 in the placenta of control group was 82.7 (29.0/0.35) and that of treatment group was 204.9 (49.0/0.24). It means that the ratio of proinflammatory to regulatory cytokine was higher in treatment group with calculation more than twice than that in control group.
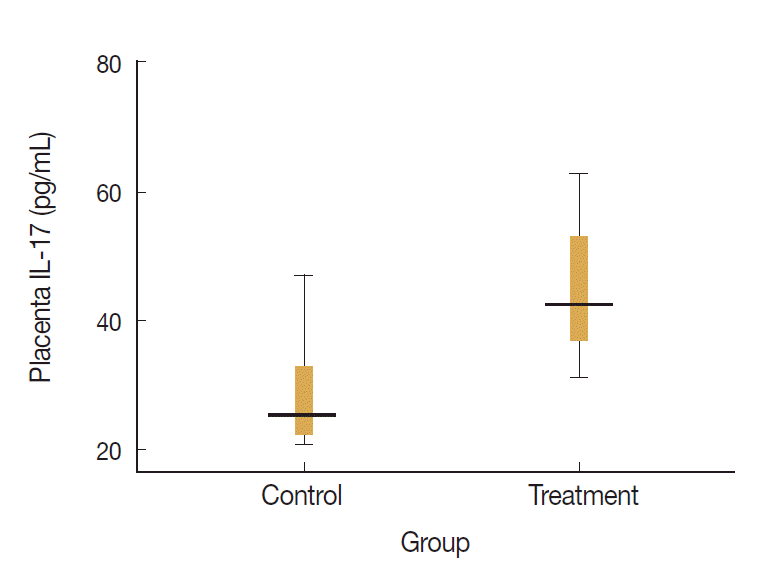
Levels of placenta IL-17 in control and treatment groups. Placenta IL-17 in the treatment group was significantly higher than that in the control group (P=0.01, unpaired t-test).
Fetal body weight
Data visualization of fetal weights of treatment and control groups are presented on the box-plot diagram (Fig. 4). Data analysis and calculation were done by using the Non-Parametric Structural Equation Modeling and the results are seen in Fig. 5.
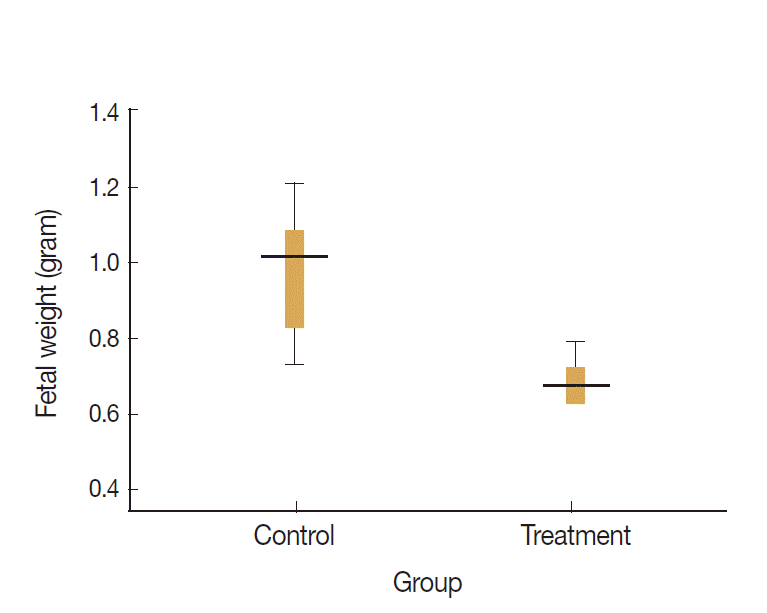
Fetal weight of control and treatment groups. Fetal body weight in mice of treated group (infected) was significantly lower than that in the control group (P=0.02, unpaired t-test).
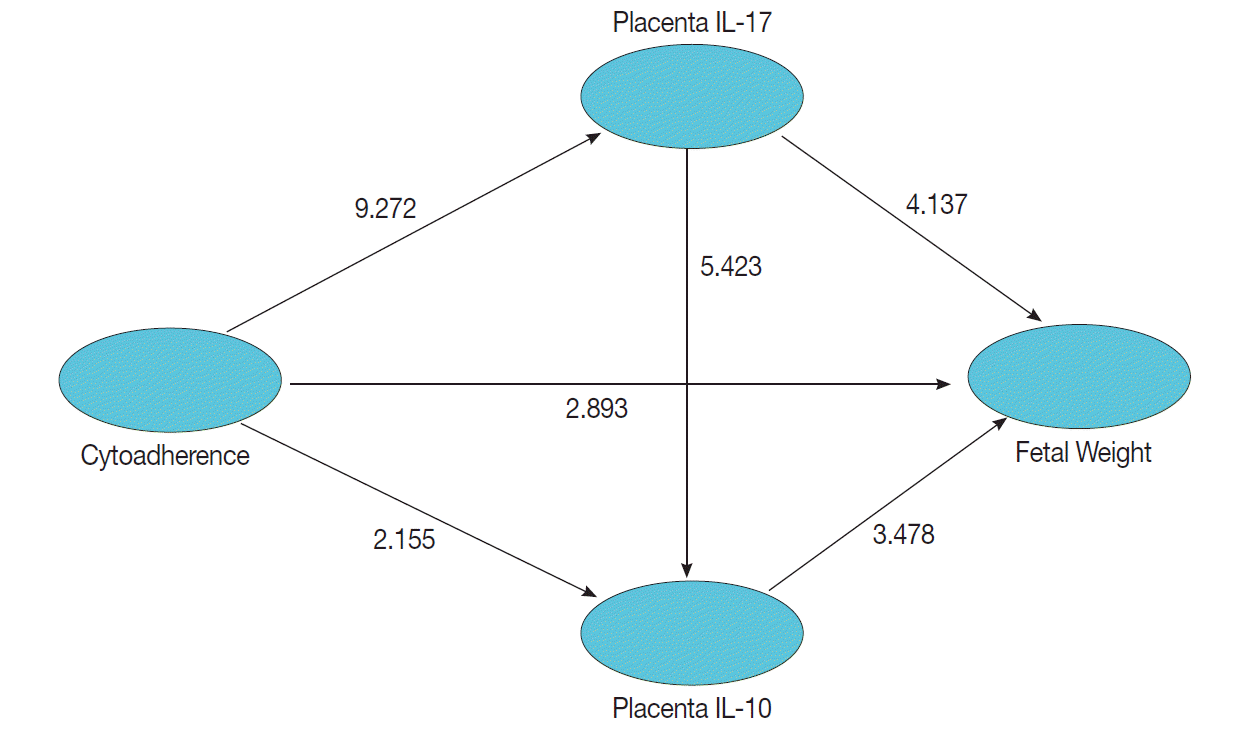
Models of structural equation modeling (SEM) for determining significance of relationship between infection by Plasmodium berghei, cytoadherence, levels of IL-17 and IL-10 in the placenta, and fetal weight.
This study set a confidence interval of 95% or P=0.05, which means a significant result was obtained when a count value is t≥1.96. Statistical analysis showed that cytoadherence caused an increase in IL-17 level in the placenta (tcount=9.272 ≥ttable=1.96; path coefficients=0.549; R2=0.301) and decrease of placenta IL-10 level (tcount=2.155≥ttable=1.96; path coefficients=-0.157; R2=0.080) (Fig. 6). Cytoadherence could also cause low fetal weight (tcount=2.893≥ttable=1.96; path coefficients=-0.178 R2=0.265). The increased level of placenta IL-17 caused low fetal weight (tcount=4.137≥ttable=1.96; path coefficients=-0.385; R2=0.265). Interestingly, low fetal weight was caused by a decrease of placenta IL-10 (tcount=3.478 ≥ttable=1.96; path coefficients=-0.242; R2=0.265), and a high IL-17 resulted in a low placenta IL-10 (tcount=5.423≥ttable=1.96; path coefficients=-0.336; R2=0.080). It can be assumed that low fetal weight 26.5% was influenced by cytoadherence, IL-17, and IL-10 with cause effect mechanism model as shown in Fig. 6.
DISCUSSION
In this study, P. berghei infection in pregnant mice caused cytoadherence of infected RBCs in the placental intervillous space with various levels. It may or may not be relevant with the degree of parasitemia although the presence of malaria parasites in pregnant women infected with malaria would lead to accumulation or sequestration of infected erythrocytes in the placental intervillous space, then leading to placental malaria [18]. The occurrence of placental malaria is caused by binding of infected erythrocytes to chondroitin sulfate A (CSA) leading to accumulation of infected erythrocytes in the placental intervillous space, infiltrated by inflammatory cells, and an increase in proinflammatory cytokines [19]. An accumulation of Plasmodium falciparum infected erythrocytes and infiltration of monocytes and macrophages, as well as alteration of the cytokine balance in placenta are important factors for the pathogenesis of adverse pregnancy outcomes [3].
In human malaria, cytoadherence occurs due to molecular interaction between the ligand P. falciparum erythrocyte membrane protein-1 (PfEMP-1) and receptor found in the placenta. Regional DBL-3-γ from PfEMP-1 encoded by var1CSA and areas of PfEMP DBL2-X-1 encoded by var2CSA are domains that are responsible for cytoadherence with CSA in the placenta [19]. A previous study provided evidence that CSA and HA, known to mediate P. falciparum adhesion to human placenta, are also involved in P. berghei infection and proposed that reduction of maternal blood flow in the placenta is a key pathogenic factor in murine pregnancy malaria [20].
Sequestration of infected erythrocytes in the placenta intervillous space is mediated by variant surface antigens (VSAs) expressed in placental malaria. In P. falciparum, these VSAPM appear largely synonymous with the PfEMP-1 family variant VAR2CSA. P. berghei as a rodent malaria does not have PfEMP-1 homologs. However, many features of murine and human placental malaria are similar, including the involvement of VSAs analogous to PfEMP-1. Thus, it appears that mouse model studies are needed to better understand the pathogenesis of malaria and VSA-dependency [21].
Infection with P. falciparum malaria during pregnancy is associated with some adverse outcomes, including fetal low birth weight due to preterm delivery and intrauterine growth retardation (IUGR) especially in primigravida [22]. Studies of placental malaria pathology through experimental models using pregnant BALB/c mice infected with P. berghei also resulted in damage and inflammation of the placenta as well as the occurrence of IUGR/LBW in the fetus [20]. Further analysis of the impact of the inflammatory response and accumulation of parasites or sequestration in the placenta and fetus may provide insight into the role of proinflammatory cytokines in the placenta pathology in P. berghei infection [23]. In this study, the infection of P. berghei in pregnant BALB/c mice as a model for malaria in pregnancy caused low fetal body weight. The result of this study also showed that during P. berghei infection the local processes at the placenta can directly cause low fetal weights. Parasite sequestration in the placenta was suspected to be a trigger of pathological conditions that caused the babies to have low body weights.
In this study, cytoadherence levels induced high levels of IL-17 and low levels of IL-10 in the placenta and directly contributed to the occurrence of low birth weight. Parasites might be directly responsible for the pathology of the placenta; however, leukocytes via the production of inflammatory cytokines associated with trophoblastic basement membrane (TBM) could cause mechanical blockage and transport of nutrients and oxygen through the placenta to the fetus [24]. Changes in TBM are associated with a high density of infected erythrocytes and infiltrating mononuclear cells in the placenta [25].
Our results showed that high levels of IL-17 in placenta during malaria caused low fetal weight. Accumulation and sequestration of parasites in the placenta tissue could induce proinflammatory immune responses [26] and tissue damage [7]. Infected placentas show an increase in inflammatory molecules, such as TNF, IL-8, and IL-6 [27]. IL-6 is a cytokine that inhibits the development of Treg cells and induces Th17 differentiation. Increased IL-6 led to an increase in IL-17 and decreased Treg cells in the uterus [9,12]. The high proinflammaproinflammatory cytokines during malaria can damage host tissues [28]. It has been reported that excessive inflammation of the placenta in the first trimester of pregnancy can cause fetal abortion [21].
Strong proinflammatory cytokine responses during placental malaria affected the growth of fetus. Previous studies also showed that the occurrence of spontaneous abortion in the first trimester of pregnancy is associated with high levels of TNF-α due to necrosis process at the site of surrounding fetal implantation [21]. IFN-γ can also increase the risk of uterine contractions and activate NK cells which induce abortion [24]. High levels of TNF-α would spur the process of infected erythrocyte cytoadherences in the capillary of placenta through binding of PfMP-1 to the CSA receptors that interfere with placental blood flow and ultimately impair fetal nutrition. If the process continues further to the second trimester, it will cause fetal growth restriction resulting in low fetal weight. Increased prostaglandin synthesis and TNF-α concentration in suspected acute placental parasitemia can also cause premature birth [14]. High inflammatory infiltration and macrophage accumulation in the placenta will disturb intervillous feto-maternal compartment, and has been identified as predictors of low fetal weight [29].
The results also showed that low levels of IL-10 may lead to placental occurrence of low birth weight in BALB/c mice infected by P. berghei. Regulation of proinflammatory cytokine production by the production of IL-10 may be a key factor that can prevent the occurrence of acute pathology [30]. This result is supported by the previous study which stated that IL-10 is a key cytokine in the protection and immunopathologic process in malaria. High levels of IL-10 observed during malaria episodes were beneficial to reduce the inflammatory response, but on the other hand could be detrimental because of decreasing in anti-parasitic cellular immune responses. IL-10 is an anti-inflammatory cytokine that acts to block the production of inflammatory cytokines produced by monocytes/macrophages such as TNF-α, IL-6, and IL-l [31].
This study has revealed that a high inflammatory cytokine as marked by IL-17 and a low concentration of IL-10 are related with low fetal weight. Result of this study showed that during malaria placenta, IL-10, an important cytokine in regulating immune system, was supressed and failed to compensate the increase of IL-17; therefore, causing an imbalance between activity of proinflammatory cytokine and regulatory cytokine. Balance ratio of IL-17/Treg in other diseases such as nephrotic syndrome showed significant increase in Th17-related cytokines (IL-17 and IL-23), and decrease in Treg-related cytokines (TGF-β1 and IL-10). The Th17/Treg ratios increased along with increased proteinuria and decreased albumin levels in patients with nephrotic syndrome [32]. An imbalance between anti-inflammatory or regulatory cytokine and proinflammatory seem to be more crucial in the pathology of placental malaria than the absolute number of the cytokine level. The result of this study supports novel Th1/Th2/Th17 paradigm in the pathogenesis of placental malaria.
Acknowledgements
The authors would like to thank Universitas Brawijaya, Malang, Indonesia for financial support for this project. We express our appreciation to the staff of Parasitology Laboratory, Faculty of Medicine, Universitas Brawijaya for the animal housing and Tarina Widaningrum SSi, MP from Biomedical Laboratory, Faculty of Medicine, Universitas Brawijaya for her invaluable assistance in ELISA assay.
Notes
We have no conflict of interest related to this work.

