Loss of infectivity of Neospora caninum oocysts maintained for a prolonged time
Article information
Abstract
The purpose of this study was to investigate whether sporulated Neospora caninum oocysts, which had been stored for 46 mo in a 2% sulfuric acid solution at 4℃, remain morphologically viable and infective to gerbils (Meriones unguiculatus). Six gerbils were orally inoculated with doses of 400 or 1,200 oocysts. Two mo after inoculation, the animals did not show any clinical signs, had no histological lesions, and were seronegative for N. caninum at 1: 50 in an immunofluorescent antibody test. PCR using the brain from each gerbil did not reveal N. caninum specific DNA. We conclude that oocysts preserved for 46 mo are not infective, despite being morphologically intact.
Neospora caninum is a protozoan parasite that infects a wide range of domestic (Dubey and Lindsay, 1996) and wild animals (Gondim, 2006). Neosporosis is the most important cause of abortion in cattle in many geographic regions (Anderson et al., 2000) and is responsible for neuropathy in dogs (Barber and Trees, 1996). Intermediate hosts of N. caninum can be infected transplacentally from dam to fetus (Dubey, 2003), or horizontally by ingestion of oocysts shed by a definitive host, such as dogs or coyotes (McAllister et al., 1998; Gondim et al., 2004c).
Mongolian gerbils (Meriones unguiculatus) have been shown to be susceptible to N. caninum infection. The parasite was successfully isolated from dogs through peritoneal inoculation of N. caninum-infected dog tissues in gerbils; isolation failed when the same tissues were inoculated into mice, guinea pigs, hamsters, and rabbits (Cuddon et al., 1992). Several passages of N. caninum tachyzoites were achieved through peritoneal inoculations of the parasite in gerbils (Gondim et al., 1999). Gerbils were also demonstrated to be susceptible to oral inoculation with N. caninum oocysts (Dubey and Lindsay, 2000; Basso et al., 2001; Gondim et al., 2001); this rodent species can be infected by a single oocyst (Trees et al., 2002). The purpose of this study was to investigate if N. caninum oocysts remain infective to gerbils after prolonged storage at 4℃ in an acidified solution.
Neospora caninum oocysts (NC-beef strain) were produced in a previous experiment (Gondim et al., 2002), and feces containing oocysts were homogenized and suspended in 2% sulfuric acid at 4℃, without purification of oocysts. In July 2004, a total of 30,000 sporulated N. caninum oocysts were purified by flotation in Sheather's solution, counted, divided in 4 plastic tubes (2 ml-size) in 2% sulfuric acid solution, and stored at 4℃. In June 2005, oocysts were washed 4 times with sterile phosphate buffer solution (PBS) to remove the sulfuric acid, and recounted. Oocysts (46-mo-old) were resuspended in sterile PBS and distributed in 6 tubes of 1 ml each. Three tubes contained 400 oocysts each, and the other 3 tubes contained 1,200 oocysts each.
Fourteen female Mongolian gerbils, 8-12 weeks old, were purchased from Universidade Federal da Bahia. Six gerbils were orally inoculated with N. caninum oocysts using an esophageal cannula. An additional 6 uninoculated gerbils served as negative controls. All animals were anesthetized in a plastic chamber with isofluorine before oral inoculation. In the test group, 3 animals were administered 400 oocysts each, and 3 gerbils received 1,200 oocysts each. The negative control gerbils were orally administered 1 ml of PBS each. After oral inoculation, animals were observed daily for clinical signs. Two mo after inoculations all animals were killed. Samples of blood and brain were collected from each gerbil. Three-fourths of the brain was used for DNA extraction and one fourth was fixed in 10% neutral buffered formalin.
A group of 3 gerbils and 3 pregnant cows that had been previously inoculated with oocysts (stored in 2% sulfuric acid at 4℃ up to 108 days) (Gondim et al., 2002; 2004a) produced by the same dogs, that originated the oocysts for the current experiment, were considered as positive controls. The 3 gerbils were orally inoculated with 30 oocysts each, and infection was confirmed in the 3 animals by serology and PCR (Gondim et al., 2002). The 3 pregnant cows received 1,500 oocysts each, and 2 of 3 cows became infected (Gondim et al., 2004a).
Two gerbils were subcutaneously inoculated with 7,500 N. caninum tachyzoites in 0.5 ml of PBS each in order to produce positive control tissues for PCR and positive sera for serology. One gerbil was killed 9 days after infection, and the other animal was killed 30 days after infection.
Pre- and post-inoculation sera were tested for N. caninum antibody using an indirect fluorescent antibody test (IFAT), as described by Dubey et al. (1988) with minor modifications. A 1: 50 cut-off dilution was employed and a commercial fluorescein isothiocyanate labeled anti-mouse IgG was used as secondary antibody (Sigma, St. Louis, Missouri, USA). IFAT reactions were only considered positive if the whole tachyzoite surface was fluorescent.
Pre- and post-infection sera were obtained from 2 gerbils, which had been subcutaneously infected with N. caninum tachyzoites, and used as negative and positive controls. Serology and PCR (described below) evaluations were performed in positive controls to demonstrate that all techniques functioned properly.
Three-fourth of the total brain was homogenized with a pestle and mortar in liquid nitrogen. DNA extraction was performed using an Easy-DNA™ kit (Invitrogen, São Paulo, Brazil). DNA from N. caninum and Toxoplasma gondii tachyzoites of NC-Bahia and RH strains were used as positive controls and ultrapure water as a negative control. A 100 bp DNA ladder (Invitrogen) was used as a marker.
PCR reactions were performed in 25 µl volumes containing the NP6/NP21 primer pair (Yamage et al., 1996), a PCR Master Mix (Promega, Madison, Wisconsin, USA), and the template. Reaction conditions were 1 cycle at 94℃ for 2 min; 40 cycles of 94℃ for 30 sec, 53℃ for 30 sec, 72℃ for 30 sec; and a final 72℃ for 5 min. A reamplification of the PCR products was done using the same conditions. PCR for T. gondii was performed similarly, except by using the primer pair TgB1-1 and TgB1-4 (Burg et al., 1989) and an annealing temperature of 55℃. PCR products were analyzed by electrophoresis on a 2% agarose gel stained with ethidium bromide and visualized under ultraviolet light.
One-fourth of each gerbil brain that was fixed in 10% neutral buffered formalin was processed for histological examination and stained with hematoxylin and eosin (H-E). Nine stained sections from each gerbil were analyzed for lesions or parasite stages.
Sporulated N. caninum oocysts which had been storage for 46 mo were orally inoculated to 6 gerbils. The animals did not show any clinical signs, had no histological brain lesions, and did not have specific antibodies at a 1: 50 cutoff by IFAT. PCR using brain from each gerbil did not reveal N. caninum specific DNA. In contrast, results of IFAT (titers of 1: 400 and 1: 6,400) and PCR (Fig. 1) were positive in the gerbils that had been inoculated with N. caninum tachyzoites, demonstrating that the procedures were sensitive enough to detect infection of gerbils. The 6 gerbils used as negative controls did not present any clinical signs or lesions and were negative for N. caninum infection by IFAT and PCR.
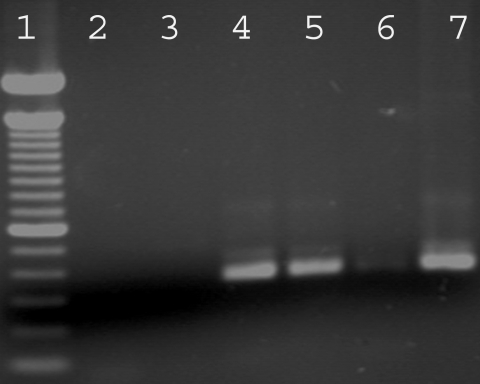
PCR for Neospora caninum using Np21-Np6 primers (Yamage et al., 1996). Lanes (1) A 100 bp DNA ladder; (2) Negative control; (3) Empty lane; (4-5) Brains from 2 gerbils infected with N. caninum tachyzoites; (6) Empty lane; (7) N. caninum DNA (positive control).
Gerbils were inoculated with 400 or 1,200 morphologically intact oocysts each. Oocysts in storage were counted in 2 occasions, immediately before inoculation and 1 year earlier. The number of sporulated N. caninum oocysts observed dropped from 30,000 to 4,800 during this interval.
Horizontal transmission of N. caninum by ingestion of parasite oocysts is associated with abortion outbreaks in cattle (McAllister et al., 1996, 2000; Jenkins et al., 2000), and has been demonstrated to be necessary to sustain N. caninum infection in bovine herds (McAllister, 1999). After dogs were identified as definitive hosts of N. caninum (McAllister et al., 1998), several studies involving experimental infection with oocysts have been done (De Marez et al., 1999; Dubey and Lindsay, 2000; O'Handley et al., 2002; Trees et al., 2002; Gondim et al., 2004a, 2004b; Rodrigues et al., 2004), however, the biology of N. caninum oocysts remains poorly understood. Before the a present experiment, there was no information regarding infectivity of N. caninum oocysts maintained for a prolonged time. Similar protozoal oocysts, such as those of T. gondii, are infective for several years in 2% sulfuric acid at 4℃ (reviewed by Dumetre and Darde, 2003).
In the present study, N. caninum oocysts, which had been stored for 46 mo in 2% sulfuric acid solution at 4℃, were orally inoculated in gerbils. The oocysts were first counted when they were 2 years old, and then recounted when they were nearly 4 years old; during this interval, the number of oocysts dropped from 30,000 to 4,800. It is possible that a portion of this reduction in oocyst numbers may have been a consequence of successive centrifugations to rinse away sulfuric acid. The oocysts appeared morphologically normal immediately before inoculation; however, they were not infective to gerbils.
The oocysts used in the current experiment were the remaining part of the oocysts used in other 2 studies (Gondim et al., 2002, 2004a). The oocysts, when used after a few weeks of storage at 4℃ in 2% sulfuric acid, were infective to gerbils that received 30 sporulated oocysts each (Gondim et al., 2002). Part of the oocysts stored up to 108 days at the same conditions, were infective to cows, which were orally administered 1,500 oocysts each (Gondim et al., 2004a).
In another experiment, Trees et al. (2002) used doses of 1, 10, and 100 N. caninum oocysts to infect 6 gerbils; all animals seroconverted and N. caninum DNA was detected in 5 of 5 tested gerbils. Because gerbils have been previously demonstrated to be highly susceptible to infection with N. caninum oocysts, yet in the present study gerbils did not become infected with oocysts preserved for 46 mo at 4℃ in dilute sulfuric acid. We conclude that N. caninum oocysts do not survive 46 mo in these conditions, however, further studies are necessary to accurately determine the exact time point when the oocysts lose their infectivity.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Paulo Maiorka for helping with histopathological analyses and Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) for the scholarship master degree to R.S. Uzeda.
References
Notes
This work was supported by FAPESB (Fundação de Amparo à Pesquisa do Estado da Bahia) and JICA (Japan International Cooperation Agency).