Species Diversity and Seasonal Distribution of Culicoides spp. (Diptera: Ceratopogonidae) in Jeju-do, Republic of Korea
Article information
Abstract
Biting midges belonging to the genus Culicoides (Diptera: Ceratopogonidae) were collected by Mosquito Magnet® and black light traps at 5 sites on Jeju-do, Republic of Korea (Korea), from May-November 2013 to determine species diversity and seasonal distribution. A total of 4,267 specimens were collected, of which 99.9% were female. The most common species was Culicoides tainanus (91.8%), followed by C. lungchiensis (7.2%) and C. punctatus (0.6%), while the remaining 4 species accounted for <0.5% of all Culicoides spp. that were collected. High numbers of C. tainanus were collected in May, followed by decreasing numbers through August, and then increasing numbers through November when surveillance was terminated. Peak numbers of C. lungchiensis were collected during September, with low numbers collected from May-August and October-November. The presence of C. lungchiensis in Korea was confirmed by morphological and molecular analyses.
Members of the genus Culicoides Latreille (Diptera: Ceratopogonidae), or biting midges, are small (0.5-2.0 mm in length) blood feeding insects distributed worldwide, with many species impacting on human and veterinary health as nuisance biters and vectors of human and animal pathogens [1,2]. The first nationwide biting midge surveys in the Republic of Korea (Korea) were reported in 1974 [3,4], with seasonal abundance and host blood meal analysis of Culicoides spp. collected from cattle and poultry farms in Gyeonggi-do reported much later [5]. More recently, a brief history of the Culicoides fauna of Korea was reported by Kim et al. [6] along with new records of 3 Culicoides spp. (C. nasuensis Kitaoka, C. pallidulus Yu, and C. jacobsoni Macfie) [7], bringing the total number of confirmed records of Culicoides spp. to 31 in Korea.
Vector-borne disease surveillance was conducted by the Animal and Plant Quarantine Agency in collaboration with the Public Health Command Region-Pacific (PHCR-PAC) and the 65th Medical Brigade to determine the species composition and seasonal and geographical distributions for Culicoides spp. and associated pathogens using Mosquito Magnet® and black light traps at Jeju Island, a special self-governing Province in Korea, from May-November 2013. The surveillance was focused on international ports on Jeju Island to address the possible risk of exotic species of vector midges being newly introduced, as suggested by reports of biting midges being transported on boats [8] and on aircraft [9].
Three Mosquito Magnet® traps (Liberty Plus Model, Woodstream Corp., Lititz, Pennsylvania, USA) and 2 black light traps (Black Hole model, BioTrap, South Korea; http://www.bio-trap.com) were used for surveillance of mosquitoes, biting gnats (Culicoides spp.), and other biting flies at the Jeju airport cargo warehouse (126.498785 E, 33.506016 N), Jeju airport detection dog center (126.504718 E, 33.510294 N), a cattle farm at Yonggang-dong village, (126.586100 E, 33.455502 N), the Yonggang animal quarantine station (126.587995 E, 33.455616 N), and the Seogwipo seaport (126.564657 E, 33.455616 N) (Fig. 1). Traps were operated continuously, and specimens were collected twice per month from May-November 2013. Midges were processed as described previously [6] and identified using the morphological keys and descriptions of Arnaud [10] and Wada [11] and the checklist for Korea [7].
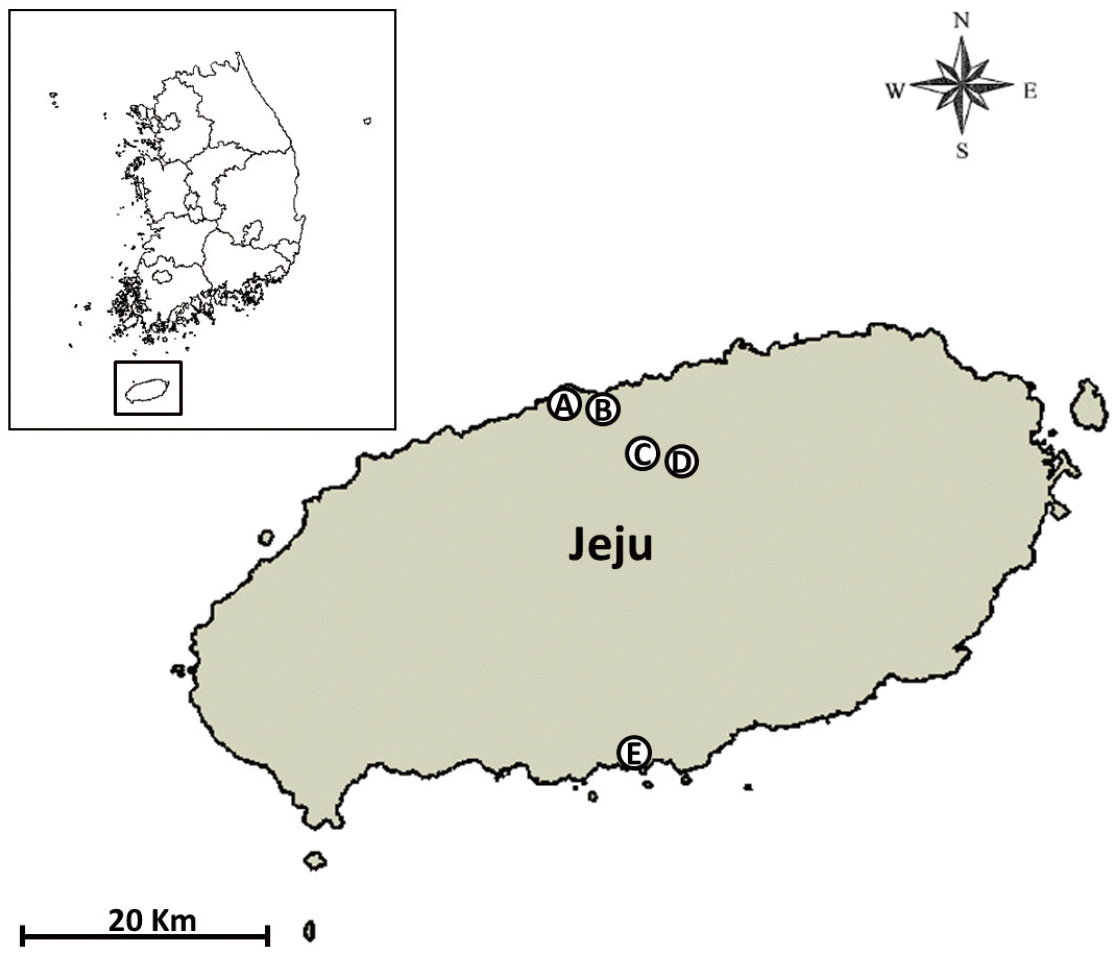
Survey sites for Culicoides spp. collected by Mosquito Magnet® and black-light traps at 5 sites from May-November 2013 in Jeju-do, a special self-governing Province, Republic of Korea (A: Airport cargo warehouse, B: Airport detection dog center, C: Cattle farm at Yonggang-dong, Jeju, D: Yonggang animal quarantine station, and E: Seogwipo port).
A total of 4,262 female (99.9%) and 5 male (0.1%) Culicoides spp., belonging to 7 species, was collected. Culicoides tainanus Kieffer was the most commonly collected species (91.8%), followed by C. lungchiensis Chen & Tsai (7.2%), and C. punctatus (Meigen) (0.6%), while the remaining 4 species accounted for 0.4% of all Culicoides spp. collected (Table 1). C. tainanus was collected only at 2 sites, a cattle farm at Yonggang-dong (3,903; 99.6%) and at the Yonggang animal quarantine station (14; 0.4%), while C. lungchiensis and C. punctatus were collected only at the cattle farm at Yonggang-dong (308 and 25, respectively). C. tainanus was most frequently collected during May, with decreasing numbers through August and then increasing numbers from August-November when collections were discontinued. These results coincided with that of Lee [2], where C. tainanus was the most common species collected at black light traps at 13 inland cowsheds (Taebaek, Yangyang in Gangwon-do, Incheon Metropolitan city, and Haenam in Jeollanam-do) in Korea. The dominance of C. tainanus at a cattle farm at Yonggang-dong, Jeju, agreed with the host preference of these species for cattle [2,12].

Total number (%) of Culicoides spp., by species and collection site, collected by Mosquito Magnet® (MM) and black-light (BL) traps at 5 sites from May-November 2013 at Jeju-do, a special self-governing Province, Republic of Korea
C. lungchiensis was collected in low numbers from May-August and November with high numbers collected during September (Table 2; Fig. 2). In contrast to the inland sites, only 2 specimens of any species were collected at the airport and none at the seaport suggesting that these sites are not conducive to the survival of Culicoides, which in turn reduces the risks for the establishment of any new species arriving at these ports.

Monthly prevalence of Culicoides spp. collected by Mosquito Magnet® and black-light traps at 5 sites from May-November 2013 in Jeju-do, a special self-governing Province, Republic of Korea
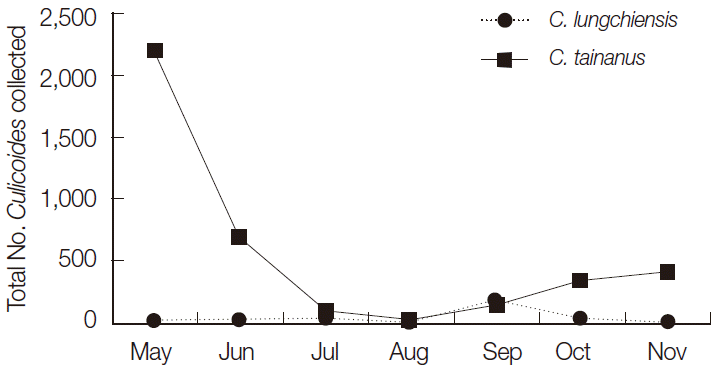
Seasonal distribution of 2 predominant species, Culicoides tainanus and C. lungchiensis collected from Mosquito Magnet® and black-light traps at 5 sites from May-November 2013 at Jeju-do, Korea.
Seasonal studies of midge populations in Korea have only been investigated in months from April-November [2,5,6,12,13], and all showed that midge populations were already active at the start of sampling period and some additionally showed that some populations, like that observed here for C. tainanus on Jeju Island, were still active and even increasing at the end of the sampling period. Consequently, the onset and cessation of midge activity in Korea is not fully understood, and complete annual surveys of midge activity are needed to clarify peak periods of activity.
Novel DNA barcode data were obtained from a wide range of Asian and Australasian species including 2 female specimens of C. lungchiensis collected from Yonggang-dong on 15 May 2013 using methods reported in Bellis et al. [14]. Neighbor-joining analysis of DNA barcode sequences [646 bp portion of the mitochondrial cytochrome c oxidase I gene (COI)] recovered from the 2 Korean specimens demonstrated 99.7-100% similarity to C. lungchiensis (N=19) from Hainan and Zhejiang provinces in China (Fig. 3). No pre-existing C. lungchiensis COI sequence accessions were available for comparison at GenBank and queries of all C. lungchiensis sequences reported here returned a closest match of 86.7-87.6% sequence similarity to C. peregrinus from Australia and China (GenBank no. KF528700, KR075719-KR075721). C. lungchiensis is morphologically similar to C. nipponensis Tokunaga, and these 2 species were reciprocally monophyletic and differed by 18.8-19.8% at COI sequence comparisons (Fig. 3). C. lungchiensis and C. nipponensis sequences reported here were deposited in GenBank (accession no. KR105376-KR105396 and KR095333 -KR095336, respectively), and associated specimen records are publically available as a dataset (http://dx.doi.org/10.5883/DS-CLUNGCHI) at the Barcode of Life Data Systems [15]. Vouchers of all of these novel specimens are lodged in the Northern Territory Quarantine Insect Collection.
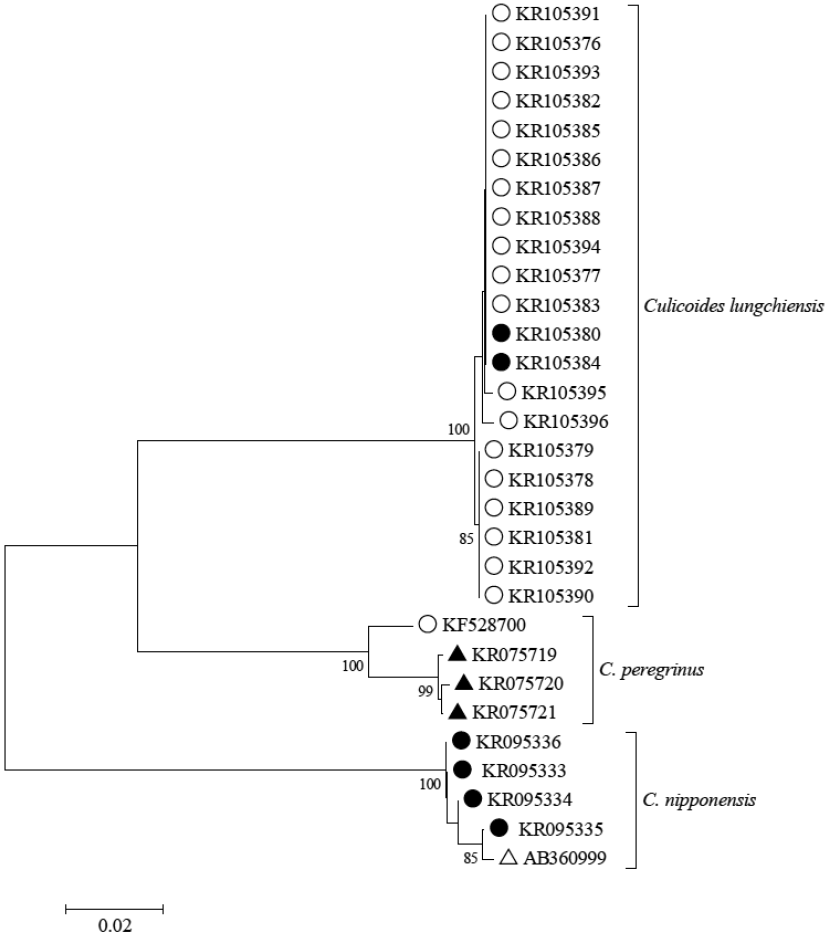
Neighbor-joining tree of COI DNA barcode sequences of taxonomically identified Culicoides lungchiensis, C. peregrinus, and C. nipponensis. Specimens from Korea and China indicated by closed and open circles, respectively; open and closed triangle indicate specimens from Japan and Australia, respectively. Scale bar equals 2% sequence difference (uncorrected). Bootstrap (10 K replicates) supports > 70% as indicated and estimated using MEGA version 6 [21]. Associated specimen records and sequence accessions available at http://dx.doi.org/10.5883/DS-CLUNGCHI.
C. lungchiensis was first described as a subspecies of C. peregrinus Kieffer [16], but was elevated to a species status by Kitaoka [17]. A range of morphological differences, including the absence of a sensory pit on the third palpal segment and the presence of only 1 pale spot in cell M4 (Fig. 4A, B) of C. lungchiensis, separates this species from C. peregrinus. The separation of these 2 species is here additionally supported by DNA barcode data (Fig. 3). Amongst the fauna of northeastern Asia, C. lungchiensis is most similar to C. nipponensis, which differs in the female mostly by the palpal ratio and the distribution of sensilla coeloconica (SCo) on the antennae and in the shape of the male aedeagus. Wada [11] also used the shape of the poststigmatic pale spot (distal margin angulate in C. nipponensis, rounded in C. lungchiensis) to separate females of these species. While this difference seems to be consistent in females, the wings of male C. lungchiensis from Lin An, Zhejiang province, China, vary in this character (Fig. 4B, C), and the distal margin of the poststigmatic pale spot of some specimens (e.g. Fig. 4C) is essentially indistinguishable from that of male C. nipponensis (Fig. 4D). Despite differences in the SCo distribution in females, males of both of these species have SCo distributed on flagellomeres 1, 11-13, and the shape of the aedeagus remains the most reliable means of morphologically distinguishing males of these 2 species.
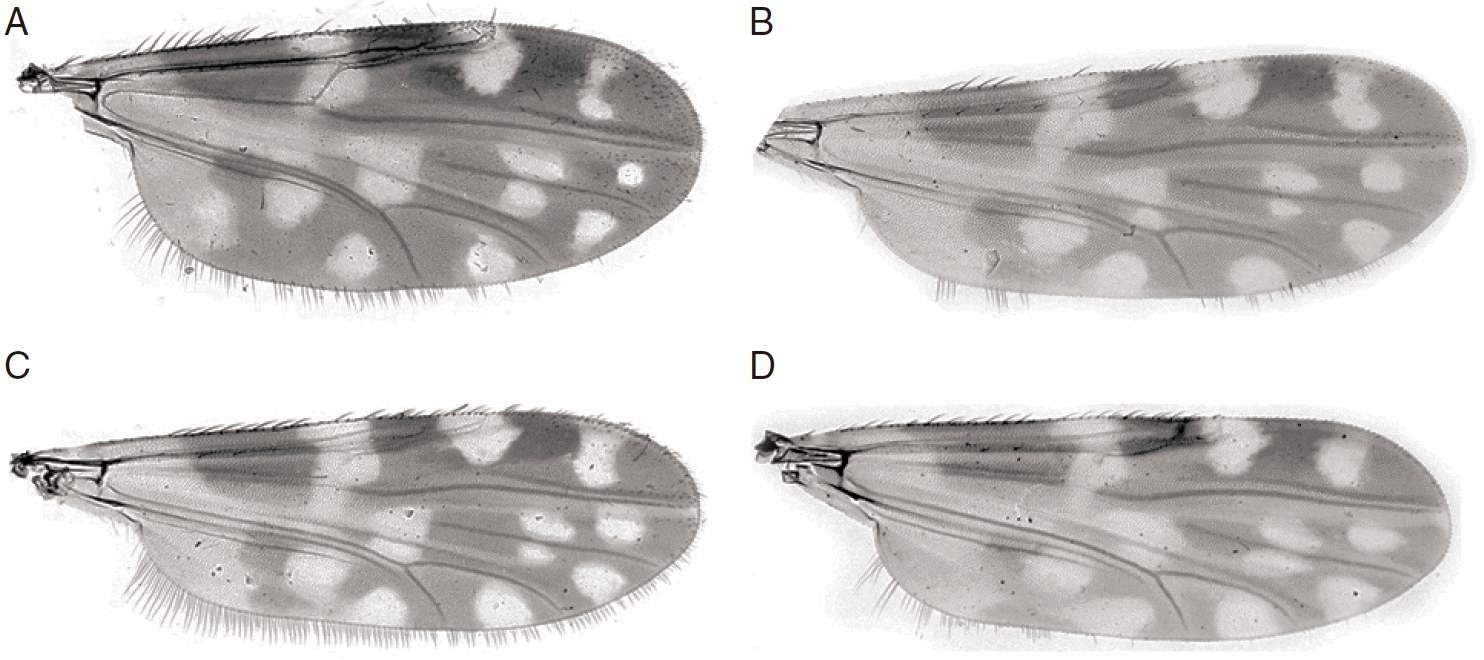
Culicoides lungchiensis Chen & Tsai. (A) Female wing. (B) Male wing. (C) Male wing variant. (D) Culicoides nipponensis Tokunaga, male wing.
The presence of C. lungchiensis on Jeju Island was confirmed by morphological and molecular analyses. This species was also reported from Japan (Honshu, Kyushu, Tsushima Is, Yakushima Is, Hateruma Is), Taiwan, and China (Liaoning, Fujian, Sichuan, Jiangxi and Yunnan, now including Zhejiang and Hainan) [11,18-20], so its presence in Korea is not surprising. Indeed, the number of specimens collected on Jeju Island is suggestive of a well established population, and it is likely that this species is also present on the Korean mainland as suggested by Wada [11]. The morphological similarity, particularly in male wing pattern, between this species and the widely reported C. nipponensis, may have resulted in specimens of C. lungchiensis being mistaken for C. nipponensis and overlooked in previous surveys. Retrospective morphological and DNA barcode analyses of mainland specimens earlier ascribed to C. nipponensis could be used to test this conjecture.
Evidence that Culicoides spp. were active when surveillance was initiated and terminated indicates the need for year-round surveillance that identifies levels of activity throughout the year. In addition, further studies on the geographical and seasonal distributions, host attraction (e.g., placement of traps near human habitation and poultry, cattle, and swine farms), temporal biting activity, pathogen infection rates, and role as potential vectors of zoonotic pathogens that impact on human and animal health are warranted. Surveillance of both endemic and potentially imported exotic Culicoides spp. and their associated pathogens are an important part of the veterinary surveillance to identify potential vector populations and their distributions throughout Korea.
Acknowledgements
This work was supported by a grant (P-1541782-2012-12-01) from the Foreign Animal Disease Division, Animal and Plant Quarantine Agency, Republic of Korea, and the Armed Forces Health Surveillance Center, Global Emerging Infections Surveillance and Response System (AFHSC-GEIS), Silver Spring, Maryland, USA, and the US Army Public Health Command Region-Pacific, Camp Zama, Japan. We thank the staff at the Jeju Regional Office, Animal and Plant Quarantine Agency for their assistance and coordination for the collection of specimens. The opinions expressed herein are those of the authors and are not to be construed as official or reflecting the views of the US Department of the Army, Department of Defense, or the US Government.
Notes
We have no conflict of interest related to this study.