Prevalence of Schistosomes and Soil-Transmitted Helminths and Morbidity Associated with Schistosomiasis among Adult Population in Lake Victoria Basin, Tanzania
Article information
Abstract
The objective of this study was to carry out a community survey on schistosomiais and soil-transmitted helminth (STH) infections in order to suggest feasible and effective intervention strategies in Lake Victoria basin, Tanzania. A total of 37 communities selected from 23 districts of the 4 regions in the Lake Victoria basin of Tanzania were involved in the study. From each of the selected locality, 50 adult community members, 25 males and 25 females, were recruited for the study. Each study participant was requested to submit stool and urine specimens. From each stool specimen, duplicate Kato-Katz thick smears were prepared and microscopically examined for Schistosoma mansoni and STH eggs. Urine specimens were processed by the filtration technique and microscopically examined for Schistosoma haematobium eggs. Ultrasound examination for morbidity due to schistosomiasis was performed. Mass treatment was done using praziquantel and albendazole for schistosome and STHs infections, respectively. Out of 1,606 adults who provided stool specimens, 199 (12.4%) were positive for S. mansoni, 349 (21.7%) for hookworms, 133 (8.3%) for Ascaris lumbricoides, and 33 (2.0%) for Trichuris trichiura. Out of 1,400 participants who provided urine specimens, 25 (1.8%) were positive for S. haematobium eggs. Because of the co-endemicity of these afflictions and their impact on vulnerable population groups, the helminthiasis could be simultaneously treated with 2 drugs, praziquantel for schistosomiasis and albendazole for STHs.
INTRODUCTION
Human schistosomiasis is a disease caused by any of 5 species of trematodes, the blood flukes, in the genus Schistosoma, and infections are acquired by contact with freshwater containing parasite larvae. World Health Organization (WHO) estimates that over 237 million people required treatment for schistosomiasis in 2010 [1] with estimates of up to an additional 779 million at risk globally [2]. About 85% of the people at risk of schistosomiasis are from Africa [3] with more than 90% of the cases occurring in sub-Saharan Africa [4,5]. King [6] estimated that schistosome infections accounted for 24-56 million disability-adjusted life years (DALYs) lost in 2010.
Two major schistosome species are prevalent in sub-Saharan region, Schistosoma mansoni and Schistosoma haematobium causing intestinal and urogenital schistosomiasis, respectively [7,8]. Both types of schistosomiasis are hyper-endemic in the Great Lake regions of East Africa, owing to the favorable habitat for snails of Biomphalaria and Bulinus genera, which are the intermediate host for S. mansoni and S. haematobium, respectively [9]. Surveys have revealed the presence of the disease in other parts of the country, including Lake Victoria [9,10]. Lakeshore communities are typically dependent on water from these lakes, rivers, and ponds for various daily activities, including cooking, bathing, and washing clothes. Fishing is usually a major occupation of men of these communities, further bringing them into contact with water [11]. Historical studies have revealed that both schistosomiasis mansoni and schistosomiasis haematobium have been endemic for a long time in Tanzania [12,13]. Through a full consideration of the amount of end-organ pathologies to the liver (in the case of S. mansoni and S. japonicum infections) and bladder and kidneys (in the case of S. haematobium infection) [4], together with chronic morbidities associated with impaired child growth and development, chronic inflammation, anemia, and other nutritional deficiencies, some new disease burden assessments estimate that schistosmiasis accounts for up to 70 million DALYs lost annually [5].
WHO estimates that more than 1 billion people are chronically infected with soil-transmitted helminths (STHs) [14]. STHs persist exclusively in the poorest populations often living in remote, rural areas, urban slums, or in conflict zones [15]. Ascaris lumbricoides, hookworms (Ancylostoma duodenale and Necator americanus), and Trichuris trichiura are the most common STH species with global prevalence of about 1,000, 900-1,300, and 500 million cases, respectively [16-18].
Of the STH species, hookworms are the most widely distributed species occurring throughout much of East Africa [19]. Both schistosomiasis and STHs have long been known to be co-endemic in the Lake Victoria basin in Tanzania [4,20,21]. A comaparable situation has been reported in the Kenya [5,22] and Uganda sides of the basin. A. lumbricoides and T. trichuria have the highest prevalences in areas close to Lake Victoria of Tanzania, western Kenya, and southeastern Uganda [19,23].
The morbidity caused by STHs is most commonly associated with infections of moderate-to-heavy intensities [24,25]. Several studies have revealed the impact of STH infections as significant predictors of protein-energy malnutrition, iron-deficiency anemia (IDA), vitamin A deficiency (VAD), and poor academic performance among schoolchildren in different countries [26-29]. Moreover, these consequences may continue into adulthood with effects on the economic productivity which trap the communities at risk of infections in a cycle of poverty, underdevelopment, and disease [30]. Hence, benefits of successful STH control programmes extend well beyond eliminating STH as they improve the nutritional and health status of children as well as contribute to higher educational attainment, labor force participation, productivity, and income among the most vulnerable populations [31-33].
Schistosomes and STH-immunosuppressive features have possible impact on promoting susceptibility to HIV/AIDS, tuberculosis, and malaria [34,35], and there is evidence that female genital schistosomiasis caused by S. haematobium may significantly increase the likelihood of contracting HIV/AIDS [22]. Moreover, there are adverse effects on pregnancy outcome and agricultural worker productivity [2,6,36-39]; helminths are strongly associated with poverty in sub-Saharan Africa. Therefore, new and ongoing efforts to control and eliminate helminths and neglected tropical diseases (NTDs) generally represent key elements for achieving Africa's Millennium Development Goals (MDGs) [2,36]. As such, the successful STH control programmes have enormous benefits of improved nutritional and health status of children for higher educational attainment, labor force participation, productivity, and income among the most vulnerable populations [33,41]. Mass drug administration (MDA) has been a major approach to control human helminthiases in developing countries [42]. In order to control schistosomiasis and STHs, WHO recommends MDA if the prevalence exceeds 10% and has the target of deworming at least 75% of school-aged children and other high-risk groups by administration with praziquantel for schistosomes and albendazole or mebendazole for STHs [22].
Conversely, control of helminth infections has been suggested as the means to facilitate control of the big 3 species [43,44], especially by reducing the frequency of malaria fevers, the frequency of severe and cerebral malaria, and the prevalence of anemia [45-47]. Furthermore, new evidence points to substantial geographic overlap between NTDs and the big 3 helminthic diseases, with emerging data suggesting that control of NTDs could actually become a powerful tool for combating HIV/AIDS, tuberculosis, and malaria [48]. The main objective of this study was to carry out a survey on schistosomiasis and STHs in order to suggest feasible and effective intervention strategies in the Lake Victoria basin, Tanzania.
MATERIALS AND METHODS
Study area and population
The study area is the basin of Lake Victoria located on the northwest of Tanzania (Fig. 1). The area stretches from the west through south to the eastern side of the lake. It is comprised with Kagera, Mwanza, and Mara on the lake shore and Shinyanga about 60 km away from the lake. The study area is bordered by Uganda on the north, and Rwanda and Burundi on the west. The main ethnic groups include Wahaya, Wasukuma, Wazinza, Wakerewe, Wajita, Wakurya, and Waluo. The major occupations of these people are peasant farming, pastoralism, and fishing. Much of the activities have a bearing to schistosomiasis and STH infections as they are performed without protective gear. The level of sanitation and hygiene in the area is as low as in any poor resource settings.

Map of the study areas. Localities in Kagera region (1. Bunena, 2. Kiziramuyaga, 3. Kyenshama, 4. Nyairigamba, 5. Buzirayombo, 6. Bwanga, 7. Bwina, 8. Runazi, and 9. Kahengere), Mwanza region (10. Tumaini, 11. Mazoezi, 12. Lumeji, 13. Nyamikoma, 14. Mwaging’hi, 15. Kigongo, 16. Bugogo, 17. Chibingo, 18. Kasamwa, 19. Nyakalilo, 20. Bungonya, and 21. Busisi), Mara region (22. Gamasara, 23. Ochuna, 24. Marasibora, 25. Minigo, 26. Mwisenge, 27. Guta “A”, 28. Nyamitwebili, and 29. Bulamba), and Shinyanga region (30. Mseki, 31. Bukomela, 32. Masumbwe, 33. Luhumbo, 34. Songwa, 35. Shishiyu, 36. Ngugunu, and 37. Sapiwi).
Specimen collection and examinations
Collection of stool and urine specimens was done at the selected schools where inhabitants visited. Each village person was given 1 stool container and 1 urine container on the first day and asked to bring the urine and stool specimens. The stool samples collected were processed to make duplicate Kato-Katz thick smears covered with cellophane soaked in glycerine and malachite green [49]. The smears were examined for hookworms and other helminth eggs or larvae within 1 hr after preparation. Examination for S. mansoni eggs were done a few minutes later. The egg counts on each of the 2 slides were added together and divided by 2 to get the mean number of egg counts.
Urine samples were collected after 10.00 a.m. to diagnose S. haematobium based on detection of eggs in urine using the filtration technique with nuclepore membranes according to WHO [21]. Urine samples were mixed thoroughly and 10 ml were drawn using a syringe and passed through the membrane where eggs were logged. Examination was done on site where detected eggs were counted and expressed as the number of eggs per 10 ml of urine.
Data management and analysis
Data entry was done using Dbase IV (Borland International, Scotts Valley, California, USA) and a double entry system was used for quality control. Data was transferred to STATA version 8 software (Statacorp 2000, College Station, Texas, USA) for analysis. Analysis was done by generation of some frequency tables, cross tabulations, and calculation of prevalence.
Ethical considerations
The ethical and scientific clearance was obtained from the Medical Research Coordinating Committee of the National Institute for Medical Research before the implementation of the study. The study team visited villages where community leaders were met and the objectives, procedure, potential harm, and benefits of the study were explained to them. The leaders in turn convened meetings of the community members and the same was explained to them before the consent to participate in the study was sought. Any participant would be free to withdraw from the study at any time of the study period when he or she felt to do so. The decision to refuse or withdraw from the study would have no negative effect on benefits provided during and after the study.
All subjects who would be found infected with schistosomiasis or intestinal helminths would be treated following standard treatment guidelines. Participants were informed that information will be confidentially kept only using code numbers instead of names of participants.
RESULTS
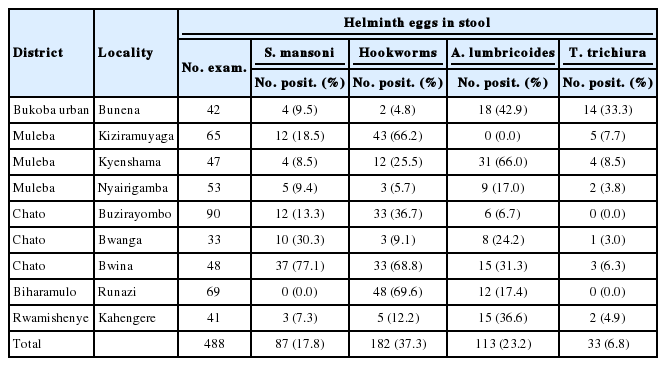
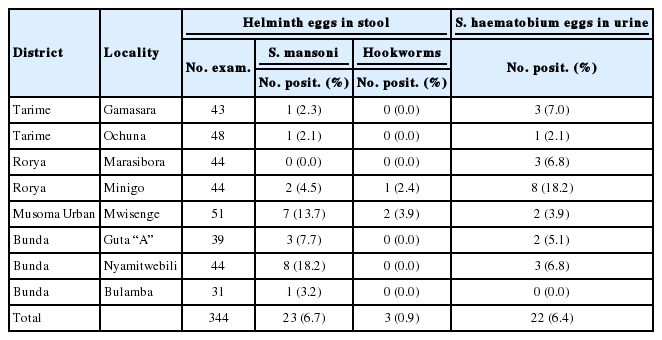
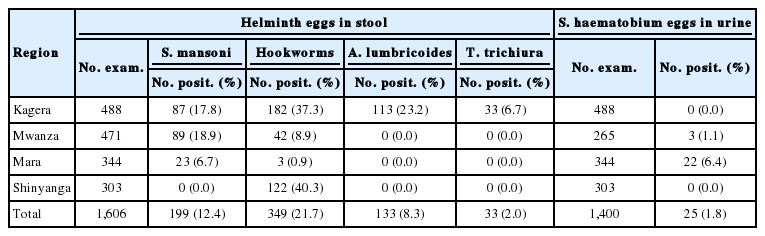
A total of 1,606 from 36 selected communities, mostly from the shoreline of the Lake Victoria basin in Tanzania, were recruited for the study (Table 1). Out of 1,606 adults who provided stool specimens, 199 (12.4%) were positive for S. mansoni, 349 (21.7) for hookworms, 133 (8.3%) for A. lumbricoides, and 33 (2.0%) for T. trichiura. A total of 1,400 adults provided urine for S. haematobium diagnosis out of whom 25 (1.8%) from Mwanza and Mara were positive for eggs (Table 1). The highest prevalence (18.9%) of intestinal schistosomiasis were recorded in Mwanza region followed by Kagera with 17.8%. No intestinal schistosomiasis was found among study participants from Shinyanga. It was only in Kagera region that all the 3 common STHs at various prevalence rates were found.
Mean prevalence of schistosomes and soil-transmitted helminths among adult population in Lake Victoria basin, Tanzania
Interestingly, no schistosomiasis haematobium patient was recorded in Shinyanga region despite its being located in the hinterland. Shinyanga is naturally the driest of the 4 regions, and the study was done in mid-July during draught when S. haematobium transmission is low.
The few adult members with urogenital infections observed in Mwanza and Mara were from wet areas with paddy fields. Such areas are good habitats for snail hosts of the genus Bulinus. By that time Bulamba locality was dry besides being close to the lake.
The study team visited a total of 9 localities in 5 out of 8 districts in Kagera region. In this region, 488 adults provided stool specimens. In total, 87 (17.8%) were positive for intestinal schistosomiasis, 182 (37.3%) for hookworms, 113 (23.2%) for ascariasis, and 33 (6.8%) for trichuriasis (Table 2). The highest prevalence (77.1%) of intestinal schistosomiasis was recorded at Bwina locality in Chato District followed by Bwanga (30.3%) and Kiziramuyaga (18.5%) in Chato and Muleba Districts, respectively. Hookworm infections were the highest at Runazi (69.6%), Bwina (68.8%), and Kiziramuyaga (66.2%) in Biharamulo, Chato, and Muleba Districts, respectively. The prevalence of ascariasis was the highest at Kyenshama (66.0%) in Muleba District, followed by an urban community of Bunena (42.9%), then Bwina (31.3%) and Bwanga (24.2%), both in Chato District. Trichuriasis was highly prevalent at Bunena (33.3%) in Bukoba Urban District (Table 2). We could not record any urogenital schistosomiasis case in this region.
The study team visited a total of 12 communities from 7 of the 8 districts of Mwanza region. Out of 471 adult participants who provided stool specimens, 89 (18.9%) had intestinal schistosomiasis and 42 (8.9%) had hookworms. No one was found to be infected with Ascaris, Trichuris, or other intestinal helminths (Table 3). Out of 265 adults who had their urine specimen examined, only 3 (1.1%) from 2 communities of Geita had urogenital schistosomiasis. About a half (51.1%) of those who presented stools at Bungonya in Sengerema had intestinal schistosomiasis. Six of 14 individuals (42.9%) at Tumaini in Ilemala and 21 out of 50 examinees (42.0%) at Nyamikoma in Magu District were found infected with intestinal schistosomiasis. Mwaging’hi in Kwimba District recorded the highest prevalence of hookworms (50.0%) followed by Tumaini (35.7%) and Lumeji (21.6%) in Ilemela and Magu Districts, respectively (Table 3).
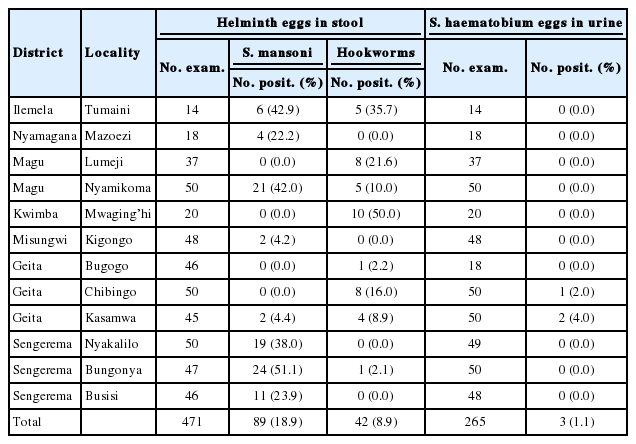
Prevalence of helminths in stool and urine samples among adult population in selected localities in Mwanza region
We recruited 344 adult members from Mara region who provided both stool and urine specimens. The region had relatively low prevalence (6.7%) of S. mansoni and even lower prevalence (0.9%) of hookworms. Only 22 (6.4%) were positive for S. haematobium (Table 4). The highest recorded prevalence of intestinal schistosomiasis in the region was from Nyamitwebili where out of 44 members who provided stool specimens, 8 (18.2%) were positive for the disease. Mwisenge locality was the second by having 7 (13.7%) positive cases for the disease. Other localities had less than 10% prevalence (Table 4). Of STHs, only hookworms were recorded at Minigo in Rorya District and Mwisenge in Musoma Urban Districts. With the exception of Bulamba in Bunda District, all communities in Mara region had cases infected with S. haematobium. The prevalence was the highest (18.2%) at Minigo community in Rorya District.
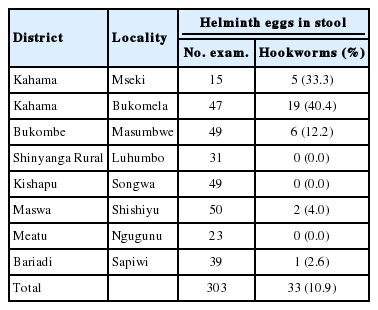
Shinyanga region had totally different findings from the other 3 regions. In this region, out of 303 members who provided both stool and urine specimens, only 33 (10.9%) were positive for hookworm eggs (Table 5). The highest prevalence for hookworms was found in Kahama District at Bukomela and Mseki localities with 40.4% and 33.3% prevalence, respectively (Table 5). No other STH infections were found. Neither could we find a person infected with intestinal or urogenital shistosomiases.
DISCUSSION
The study results showed that generally the prevalence of S. mansoni, S. haematobium, and STHs is widespread in the study area. This observation agrees to the findings of earlier studies in the area [36,50]. However, the prevalence of S. mansoni in Kagera region was very low with the exception of a few pockets in Chato District. This could be attributed to the fact that lake shores in Kagera region are open ones instead of bays such that when the wind blows makes the waves splash the shores making the place an unconduncive habitat for snail intermediate hosts for schistosomes. Moreover, some of the shores in Kagera region are formed by escarpments thus deep and less habitable by the snails. The situation is quite different from shores in Chato District in the southwest of Lake Victoria which are mainly shallow and in bays thus not susceptible to strong winds and can habor the snails. The relatively higher prevalence of ascariasis and trichuriasis in Kagera region than in other regions could be attributed to the difference in soil temperature, altitude, soil type, and rainfall [51-53]. Kagera region is wet almost throughout the year as compared to the rest of the regions in the area. The highest prevalence of S. mansoni in Kagera region that was recorded at Bwina community could be attributed to the location of the study site. Bwina ward is a thin peninsular with an average width of less than 2 km. As such, most of the area is surrounded by Lake Victoria water that is inhabited by the snail intermediate host Biomphararia species for S. mansoni. Bwanga and Kiziramuyaga localities are close to the lake area that has small bays that are conduncive areas for the snail hosts. This has an implication of the region having the best breeding ground of the STHs as well as more people consuming more vegetables that may be carrying the helminth eggs than those from other areas. The absence of urogenital schistosomiasis in Kagera could be due to an absence of ponds and streams during the time of the study, and the fact that no paddy cultivation is practiced in the area.
The high prevalence of intestinal and urogenital schistosomisiasis in the study area was a function of the distance from Lake Victoria; the former being more prevalent at localities close to the lake, whilist the latter is more so away from it. Similar observations had been reported elsewhere in East Africa [17,19,28,29,54]. The high prevalence of intestinal schistosomiasis at Nyamitwebili and Mwisenge localities could be associated with their being at the lake shore. Minigo had a lot of paddy fields that are good habitats for snail intermediate hosts for S. haematobium. The distribution of S. haematobium and S. mansoni along Lake Victoria is largely related to the distribution of the intermediate hosts [53]. Along the shore of the lake, members of the genus Biomphalaria are common [54,55] with populations living along the lake shores and islands being highly affected by S. mansoni, as the risk of infection increases [56].
In Mara region, the distribution of S. mansoni was similar to that of Mwanza with more S. mansoni close to the lake and S. haematobium on the hinterland. Moreover, in areas where S. haematobium was high, there was a significant prevalence of hookworms. The findings are in line with the ones from a previous study in Magu District in Mwanza region [35]. The spatial distribution of S. haematobium and hookworm is in accordance with the presence of ponds and streams as well as wetness and warmth of the soil in the reference area that are prerequisite for proliferation of the 2 helminths.
We could not easily establish why there were no infections due to A. lumbricoides and T. trichiura in Mwanza region or intestinal schistosomiasis in Shinyanga region. A major reason could be geographical as stated above or there were treatment programmes prior to this study.
The study results showed that schistosomiasis and 3 STHs infections (hookworms, Ascaris, and Trichuris) were co-endemic in Lake Victoria basin in Tanzania with a high probability of polyparasitism in the study participants. The prevalence of S. mansoni, S. haematobium, and STHs ranged from low to moderate in most parts of the study area. Intestinal schistosomiasis was prevalent along the Lake Victoria shores and decreased with distance from it. Conversely, the prevalence of urogenital schistosomiasis increased with distance from the Lake. The distributions of both schistosome and geohelminth infections have important implications for integrated intervention including periodic community deworming programmes, provision of safe water supply, and proper health education relevant to good personal hygiene and good sanitary practices. These activities will facilitate the reduction of both prevalence and intensity of infections of schistosomiasis and STHs in such endemic communities. Pre- and post-intervention studies to assess knowledge, attitude, and practices of the concerned population cannot be overemphasized.
Acknowledgements
The authors are very grateful to the people of Kagera, Mwanza, Mara, and Shinyanga for their tireless commitment to participate in the study for all 5 years running. Our heartfelt thanks go to the Government of the Republic of Korea through the Korea international Cooperation Agency (KOICA). Good Neighbors International (GNI) funded the study and provided expertise for conduction of the study from the beginning to its logical conclusion. Particular gratitude is extended to the staff of NIMR, Mwanza Research Center, and entire members of GNI Tanzania Western Chapter for their material, moral, and technical support during the study. We would like to thank all those who supported us in one way or another for their commitments and deligency during all the stages of the study.
Notes
The authors declared no conflict of interest whatsoever.