Plasmodium knowlesi as a Threat to Global Public Health
Article information
Abstract
Malaria is a tropical disease caused by protozoans of the Plasmodium genus. Delayed diagnosis and misdiagnosis are strongly associated with higher mortality. In recent years, a greater importance is attributed to Plasmodium knowlesi, a species found mainly in Southeast Asia. Routine parasitological diagnostics are associated with certain limitations and difficulties in unambiguous determination of the parasite species based only on microscopic image. Recently, molecular techniques have been increasingly used for predictive diagnosis. The aim of the study is to draw attention to the risk of travelling to knowlesi malaria endemic areas and to raise awareness among personnel involved in the therapeutic process.
INTRODUCTION
Malaria still remains a serious public health challenge in the tropics. It is a protozoan disease caused in humans by 5 species of the Plasmodium genus; P. falciparum, P. vivax, P. malariae, P. ovale, and P. knowlesi [1]. Malaria parasites are transmitted by female mosquitoes belonging to the Anopheles genus. Males of Anopheles mosquitoes feed only with plant juices and nectar and cannot transmit malaria [2]. Malaria was eliminated in Europe, but imported malaria is still an important health problem [3]. Plasmodium is a cause of very severe disease and often death in infants, children, and in naive adults, but causes only mild disease in adults living in endemic areas. People from malaria endemic regions, who live abroad for a period of time, become again susceptible to severe malaria after coming back to endemic areas. It suggests that there exists a kind of short lasting immunity [4]. Persisting semi-immunity does not protect against infection, but it seems to protect against severe clinical course, complications, and death.
In recent years, P. knowlesi became an important Plasmodium species infecting humans mainly in Southeast Asia. The aim of this study is to draw attention to the risk of traveling to knowlesi malaria endemic areas and to raise awareness among personnel involved in the therapeutic process.
GENERAL EPIDEMIOLOGY AND TRANSMISSION OF HUMAN MALARIA
Malaria is one of the most widespread diseases in the world and is endemic through tropical and subtropical regions. During the past 2 decades, the international community has invested heavily in malaria control. However, it is still the most widespread hemoparasitic disease in the world [5]. The malaria-endemic areas involve 108 countries inhabited by roughly 3 billion people, representing over 40% of world population [1,6]. P. vivax and P. falciparum are responsible for 80-95% of all malaria cases worldwide [7]. Falciparum malaria, which manifests itself in severe progress, occurs in 87 malaria-endemic countries, including 43 African countries [3]. It is estimated that there are about 500 million clinical cases of malaria annually with mortality up to 2.7 million per year [5]. Only in 2012, there were estimated 207 million cases of malaria, causing approximately 627,000 deaths [8]. More than 85% of malaria cases and 90% of malaria deaths occur in sub-Saharan Africa, mainly in young children [6]. Malaria elimination is the main goal of many countries, and large reductions in malaria prevalence have been achieved [9].
HUMAN KNOWLESI MALARIA
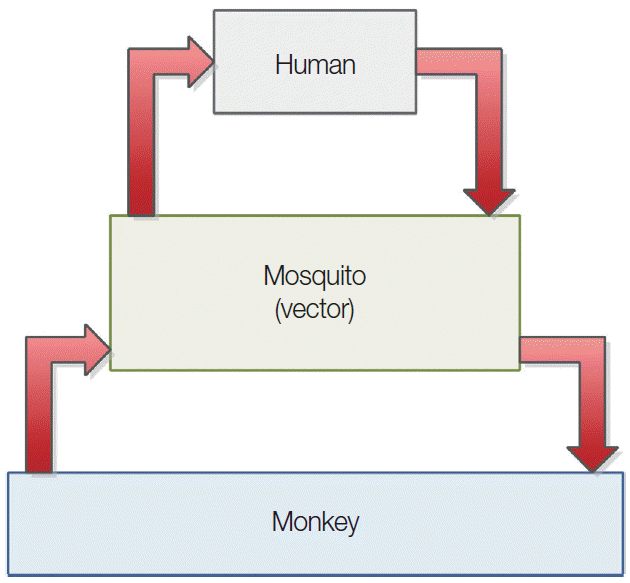
P. knowlesi has been named in honour of Robert Knowles, who first described this Plasmodium species in 1931 as a parasite of vertebrate hosts: long-tailed macaques (Macaca fascicularis) and pig-tailed macaques (Macaca nemestrina) in Southeast Asia [10]. In 1965, naturally acquired human knowlesi malaria infection was first reported in an American tourist travelling through Malaysia [11]. P. knowlesi is a natural parasite of both M. fascicularis and M. nemestrina and has a relatively broad host range extending to humans, in whom it causes zoonotic malaria [12,13]. Although transmissions and infections with P. knowlesi are mainly observed in animals, recently a great number of human cases have been identified in Southeast Asia. It has been identified as the fifth human malaria species. Knowlesi malaria is the main cause of admissions for malaria in certain hospitals and can lead to fatal infections in endemic areas [12,14,15]. Human knowlesi malaria cases have been reported in East and West Malaysia and in other countries in Southeast Asia, including Thailand, Myanmar, Singapore, Philippines, Vietnam, and Indonesia [16]. People living in the forest fringe areas and working in forests as well as tourists intending to visit the forests in Southeast Asia are at risk of knowlesi malaria [17]. Most of infections are reported in the rural jungle areas of the Malaysia peninsular and in Borneo (Sabah and Sarawak Province), where both infectious hosts (macaque and leaf monkeys) and vectors (Anopheles mosquitoes) exist predominantly [18]. Fig. 1 illustrates malaria transmission cycle and human-monkey host interaction [19].
Macaques, which colonized Asia more than 5 million years ago, were the most likely hosts during the initial emergence of P. knowlesi in this region [20]. The majority of transmission is sustained and driven by the macaque population and is dependent on relative mammalian host densities [21]. There is a greater transmission intensity of this parasite by the vectors among wild macaques, than from macaques to humans [20]. The current restriction of P. knowlesi to a vector which prefers the forest fringe habitat rather than a completely anthropophilic one has limited the emergence of P. knowlesi as a fully human malaria parasite and public health threat [21]. However, humans can acquire knowlesi malaria when they visit the forest habitat of macaques and mosquito vectors. Moreover, it is possible that current deforestation and environment changes, with associated increase of the human population, may alter the parasite, macaque host, and mosquito population dynamics and lead to an adaptive host-switch of P. knowlesi to humans [19].
Like P. falciparum, P. knowlesi can invade erythrocytes in every stage of life, not only very young ones as P. vivax and P. ovale or old erythrocytes as P. malariae [11]. As the intra-erythrocytic cycle is only 24 hr, P. knowlesi may cause rapid clinical deterioration, and in almost 7% of cases it has a severe, life-treating course with multiorgan failure (as in falciparum malaria) [11]. Studies demonstrate seasonal variations of all Plasmodium species, with maximum notifications for P. knowlesi occurring in June [9]. According to some studies, one infective bite is probably adequate to infect a monkey, while human studies have shown that volunteers exposed to 2 bites of infected mosquitoes do not always become infected [22].
Multiple infections represent a potentially greater risk to human health. Single Anopheles mosquito carrying sporozoites of 3 Plasmodium species, P. knowlesi, P. falciparum, and P. vivax, was discovered in southern Vietnam [23]. The course of infection with P. knowlesi is dependent on the host. In its natural host, infection results in prolonged low-level parasitemia, whereas in the other hosts parasitemia rise rapidly, and the infection is responsible for severe complications and may be fatal [12]. Yet so far, no evidence of man-to-man transmission of P. knowlesi has been found, and this species is considered enzootic [1].
Malaysia has a long history of malaria control programs dating back to the early 1900s, with an initial focus on environmental management techniques [9]. Although Malaysia has achieved impressive success in controlling malaria over recent decades, knowlesi malaria still constitutes an important public health problem in this country [24,25]. Lee et al. [20] examined the blood samples of 108 wild macaques in Malaysian Borneo and confirmed high prevalence of P. knowlesi (78%). Notifications of P. malariae/P. knowlesi human infections increased from 703 in 2011 to 996 in 2013 in some regions of Malaysia [24]. P. knowlesi is a main cause of hospital admissions for malaria in Kapit Hospital (Kapit, Malaysia) with more than 90 knowlesi malaria admissions per year [20]. However, actual annual incidence of this malaria may be higher, because not all people suffering from malaria have sought treatment in hospitals, and there may be asymptomatic infections or misdiagnosed cases [20].
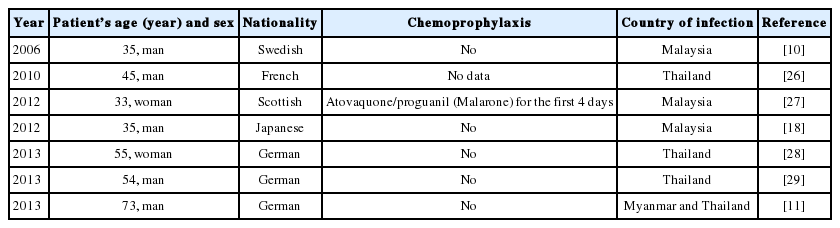
Knowledge on P. knowlesi is especially important for medical personnel in geographically contiguous countries where the possibility of spread of this infection is the largest. Medical staff working not only in endemic areas should be aware of potential risk of knowlesi malaria incidents in European travellers. Numerous case studies show increased knowlesi malaria incidents in people coming back from Southeast Asia (Table 1). Most reported cases of knowlesi malaria visited rainforest region prior to manifestation of malaria [11].
DIFICULTIES IN IDENTIFICATION OF MALARIA PARASITES
Clinically, P. knowlesi can be difficult to diagnose [27]. Timely diagnosis and treatment are crucial for reducing the risk of malaria complications [8]. For this reason, accurate identification of Plasmodium species is fundamental for proper clinical management and control strategies. Plasmodium species infections are typically diagnosed by microscopic examination of stained blood films, but there are limitations in sensitivity and specificity [15]. Although there are newer techniques, manual microscopic examination of peripheral blood smears by thick and thin film is “the gold standard” for malaria diagnosis and species identification [30,31]. The thick film should be used for the detection of malaria parasites and the thin film for identification of species. All malaria films should be examined by 2 trained observers simultaneously or subsequently. During microscopy, a minimum of 200 oil immersion fields (×100 objective) should be examined in the thick film. This procedure takes about 10 min for an experienced observer, but longer for those who not often examine films containing malaria parasites [32]. The specific changes of the shape of the occupied erythrocytes, presence of some characteristic dots (Schüffner’s dots, Maurer’s clefts, and Ziemann’s strippling) and the different morphology of the parasites in the life-cycle-stages are the basis of identification of malaria species during microscopic examination (Fig. 2) [31].
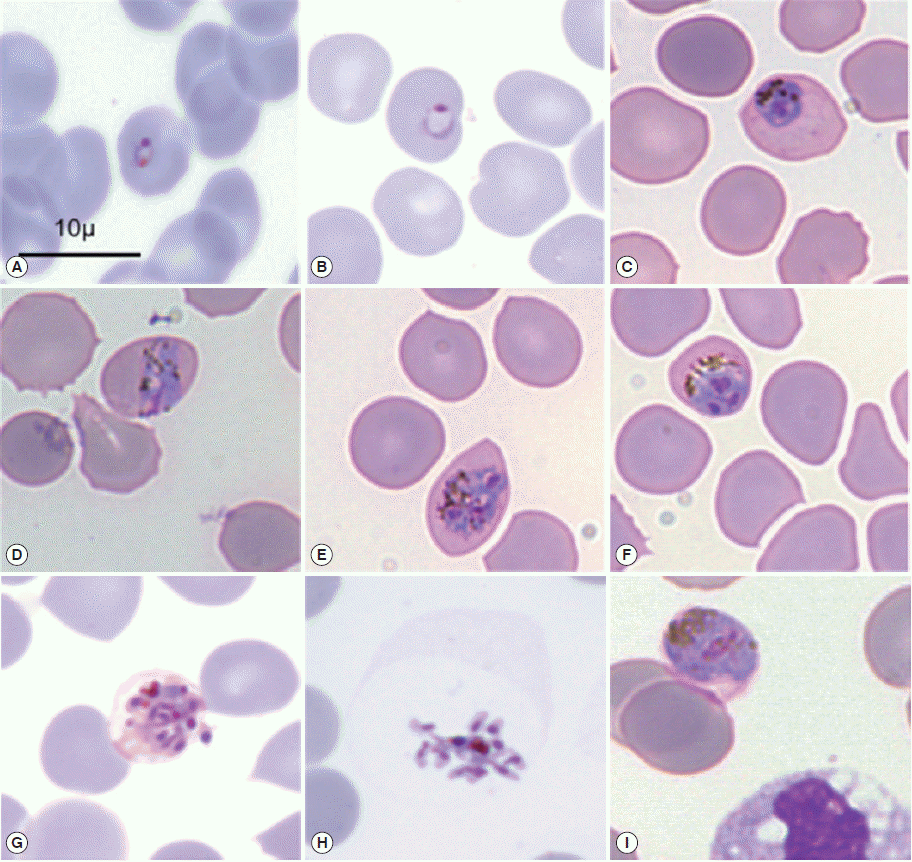
Microscopic morphology of Plasmodium knowlesi in May-Grünwald-Giemsa stained thin blood smear. Infected erythrocytes were not enlarged with the presence of much hemozoin and lacked stippling. (A, B) Young trophozoites, (C) late trophozoite, (D) equatorial band form, (E, G) schizonts, (H) merozoites, (I) gametocyte (reproduced from [26]).
The precision and reliability of microscopic examinations depends largely on the quality of stained blood films. Moreover, it is difficult to detect mixed infections or low parasitemia cases using this conventional method, especially for those involving P. knowlesi [8]. UK National External Quality Assessment Service surveys indicated that there are still encountered problems in malaria diagnosis, such as inaccurate calculation of parasitemia, failure to estimate it altogether, difficulties in distinguishing between P. vivax and P. ovale, reporting the presence of malaria parasites when none were present, and misidentifying P. falciparum as another species [32]. P. knowlesi has been frequently misdiagnosed as P. malariae via conventional microscopy due to similarities in morphology at certain asexual stages. Indeed, diagnoticians during microscopic examinations of the asexual stages of Plasmodium on thick and thin blood film are unable to distinguish early trophozoites of P. knowlesi from P. falciparum and late erythrocytic stages from P. malariae, thus the application of specific molecular assays is necessary [8,9,12,14,27].
Although microscopy is the only method of malaria detection in hospital laboratories in Malaysia, the decrease in diagnostic discrepancy is due to the increased awareness about P. knowlesi infections among the local laboratory technicians and microscopists [8,9,33]. Coupled with the fact that P. malariae infections are not frequent in Malaysia, the detection of malaria parasites with morphology similar to that of P. malariae should be reported as “P. knowlesi” [8]. Thereby, knowlesi malaria has been accounted for more than a half of all cases of malaria in Malaysia [8].
Use of molecular methods has transformed the epidemiology of malaria and highlighted infection in humans by P. knowlesi [14]. The need of use of accurate methods in characterization of malaria parasites has been confirmed by the research of William et al. [9]. When PCR was performed on the earliest P. malariae slides available, taken in 1996, 97% of tested samples were positive for P. knowlesi, with only 1 being positive for P. malariae. Yusof et al. [8] in their preliminary studies also detected P. knowlesi by PCR in almost all infections previously demonstrated as P. malariae. Molecular biology methods facilitated the identification of significantly more cases of infections caused by Plasmodium species other than P. falciparum and more cases of mixed infections than microscopic examinations [12,15]. Calderaro et al. [14] confirmed that real-time PCR assays specific for P. knowlesi are powerful diagnostic tools able to provide a novel insight into the epidemiology of malaria infections in non-endemic areas. According to UK National External Quality Assessment Service, a patient infected with parasites thought to be P. malariae, who has travelled in the Asian-Pacific region should be urgently referred to Malaria Reference Laboratory for P. knowlesi examination by PCR [32]. Although molecular diagnostic techniques can detect numerous infections that would be otherwise missed [34], these methods are slower and more expensive than microscopy, so they will not replace routine microscopy in rural hospitals where most number of malaria patients are admitted [8,9,33].
In accordance with guideline on laboratory diagnosis of malaria [32], in all cases of suspected malaria, high quality thick and thin films should be prepared, regardless of the results of immunochromatographic rapid diagnostic tests (RDTs). World Health Organization has produced RDTs indicated to confirm the presence or absence of P. falciparum assessed on a blood film. It is particularly useful when there is a relatively unexperienced observer, when pressure of work out-of-hours prevents adequate microscopic assessment or in hospitals where examination of films for malaria parasites is performed infrequently [32]. Foster et al. [15] determined parasitemia by microscopy, detected the Plasmodium species by PCR and evaluated 3 different RDTs. They noted low sensitivity of detection of P. knowlesi by all examined RDTs. They also indicated cross-reactivity, common between P. knowlesi-infected blood and both the P. falciparum and P. vivax. For this reason, patients with knowlesi malaria may be misdiagnosed with this kind of tests as infected by P. falciparum or P. vivax. Therefore, using RDTs for the diagnosis of malaria in areas where P. knowlesi is the predominant species should be proceeded with careful consideration because false-negative results may occur [10,15,32]. In patient who returned from a 2-week holiday in Sarawak, Malaysian Borneo, RDT was negative, but the nested PCR assay and molecular characterization confirmed that the patient was infected with P. knowlesi [10]. In the first described case of P. knowlesi in a Scottish traveller, despite negative antigen testing results, Cordina et al. [27] confirmed knowlesi malaria by using a PCR-sequencing assay.
MALARIA TREATMENT AND CONTROL
Delayed diagnosis is strongly associated with higher mortality, indicative of lack of malaria awareness among both patients and general practitioners [3]. Imai et al. [21] found that rapid treatment of infected individuals is the most effective in reducing infection prevalence among humans, with a 95% reduction if cases are treated quickly. Knowlesi malaria causes a wide spectrum of symptoms [35]. Natural P. knowlesi infection may progress as a self-limiting malaria with spontaneous cure, although generally the presentation progresses as moderate or can be severe, requiring anti-malarial therapy [30]. P. knowlesi is equally dangerous as P. falciparum, which is the cause of most number of deaths from malaria in the world. When treated promptly with effective antimalarial drugs, uncomplicated falciparum malaria has a mortality of roughly 0.5% [6]. Most cases of knowlesi malaria are uncomplicated and respond promptly to treatment, but approximately 1 of 10 patients develops potentially fatal complications [35]. Whenever P. falciparum or P. knowlesi is detected, the percentage of parasitized cells should be quantified and reported promptly to the responsible clinical staff, as the severity of parasitemia may affect the choice of treatment [32].
There is no classification of severe knowlesi malaria but classification of severe malaria caused by P. falciparum is currently used. Clinical manifestations or laboratory finding of severe malaria include prostration, impaired consciousness, respiratory distress (acidotic breathing), multiple convulsions, circulatory collapse, pulmonary oedema, abnormal bleeding, jaundice, hemoglobinuria, and severe anemia [36]. In addition, any case demonstrating “P. malariae-like parasites” should be treated presumably as P. knowlesi infection while waiting for the molecular confirmed diagnosis [8]. Physicians should be aware that knowlesi infection is an important differential diagnosis in febrile travellers, with a recent travel history to forested areas in Southeast Asia, including short-term travellers who tested negative with RDTs [37].
Nowadays there are geo-positioning, temporal, and spatial analysis used to identify high-risk areas and density of knowlesi malaria cases in locations with reported infections. Unless more information is obtained on the vectors as well as macaque involved in the transmission, it will be difficult to plan effective control strategies. The utilization of modern analytical tools is crucial in estimating hotspot areas for target control strategies [25]. Human malaria has been reduced or is in the process of being eliminated and simultaneously knowlesi malaria is on the rise in affecting humans. From the public health point of view, it is difficult to explain to the people that malaria has been eradicated when cases are still occurring in larger numbers than before [25]. The possibility of elimination of knowlesi malaria is limited, because forests that harbour Anopheles mosquitoes and macaque monkeys will remain a reservoir for the zoonotic transmission of P. knowlesi [23]. Vythilingam et al. [25] suggest that cases of knowlesi malaria will be on the increase in coming years, hence the need for multi-disciplinary research to combat simian malaria affecting humans.
CONCLUSIONS
To summing up, it is crucial to realize that malaria may pose a risk to any patient with an exposure history, even if travel was a long time ago. Experience in the diagnosis and treatment of malaria has fundamental role. Using of molecular methods in detection of various malaria species is crucial to avoid mortality due to malaria parasite misidentification.
Notes
We have no conflict of interest related to this work.