Infections of Larval Stages of Dicrocoelium dendriticum and Brachylaima sp. in Brown Garden Snail, Helix aspersa, in Turkey
Article information
Abstract
The aim of this study was to determine the presence and prevalence of larval stages of Dicrocoelium dendriticum and Brachylaima sp. in the first intermediate host, a species of land snail, Helix aspersa, in Turkey. A total of 211 snails were collected in April-May 2014 from pastures in Mersin District. Larval stages of D. dendriticum were identified under a light microscope. Hepatopancreas from naturally infected H. aspersa snails were examined histologically. The prevalence of larval stages of D. dendriticum and Brachylaima sp. in H. aspersa snails was found to be 2.4% and 1.9%, respectively, in Mersin, Turkey. Cercariae were not matured in sporocysts at the beginning of April; however, it was observed that cercariae matured and started to leave sporocysts by early-May. Thus, it was concluded that H. aspersa acts as an intermediate host to D. dendriticumin and Brachylaima sp. in Mersin, Turkey. A digenean trematode Brachylaima sp. was seen for the first time in Turkey.
The brown garden snail, Helix aspersa Müller, 1774, which is serving as the first intermediate host for Dicrocoelium dendriticum (Rudolphi, 1819) Looss, 1899 (Digenea: Dicrocoeliidae) and Brachylaima (Dujarjin, 1843) sp., distributed in Western Europe, Britain, and along borders of the Mediterranean and Black Sea. It has been introduced into New Zealand, Australia, South Africa, Mexico, Chile, Argentina, Haiti, and the Atlantic Islands [1].
The lancet liver fluke, D. dendriticum, lives in the bile duct and gall bladder of domestic and wild ruminants; however, it rarely affects other animals and humans with low specificity [2-4]. The life cycle of D. dendriticum involves several land snails as the first intermediate host, miscellaneous species of ants as the second intermediate host, and especially ruminants as the definitive host. The larval stages of D. dendriticum evolve in about 3-4 months in snails from the miracidia which pass into the snails with the eggs, to first and second generation sporocysts. Numerous cercariae develop in the second generation sporocysts [5,6]. It was initially reported that H. aspersa serves as a first intermediate host for D. dendriticum in the vicinity of Izmir, Turkey. It has also been reported that the second generation sporocysts and cercariae were common with the rate of 0.97% [7].
Dicrocoeliosis is a widespread parasitic disease in grazing animals worldwide. The infection is common in Europe, Asia, North Africa, and America, where the local conditions are suitable for particular species of earth snails and ants as intermediate hosts [8]. The infection is also common in sheep [9-13], goats [10,12,14], cattle [15], equids [16,17], rabbits [18], and humans [19-21] in Turkey. The disease causes severe economic losses, in terms of milk and meat production, due to liver function impairment. It can be fatal on rare occasions [2,4].
Brachylaima (Dujarjin, 1843) spp. are digenean trematodes of mammals and birds. Land snails serve as the first and second intermediate hosts [22]. Brachylaima spp. have been previously reported from various countries around the world. It has been reported that the genus Brachylaima contains at least 72 species of which only one is zoonotic [23]. Brachylaima cribbi has been demonstrated in Australia, where 3 human cases have been reported [24,25]. The presence of larval stages of Brachylaima in edible snail species is important for food hygiene. It has been already known that the brown garden snail, H. aspersa, is the first intermediate host of Brachylaima spp. in Australia and Spain [22,26-28].
Although the larval stages of D. dendriticum have been previously reported, Brachylaima spp. have not been studied in Turkey. Thus, this study aimed at determining the presence and prevalence of both trematode species in the brown garden snail.
A total of 211 snails, H. aspersa (Fig. 1A), were collected from the pastures rearing sheep with dicrocoeliosis in Mersin District, Turkey. Among them, 101 snails were collected in April, and 110 were collected in May 2014. Snails were anesthetized by injection of a sterile 50 mM magnesium chloride (MgCl2). Anesthetized snails were dissected by removing their shell. The internal organs were then inspected for the presence of sporocysts and cercariae using stereomicroscope (Nikon-SMZ800). Particular attention was given to the digestive gland and hepatopancreas. The larval stages were identified under the light microscope (Nikon-Eclipse 80i - DS-5M-L1). The hepatopancreas and digestive gland were removed from some infected snails with larval trematodes, and they were fixed in 10% buffered formalin for 24-48 hr. The histopathological samples (4-5 μm in thickness) of snails were observed under a light microscope after the standard making process of histological samples.
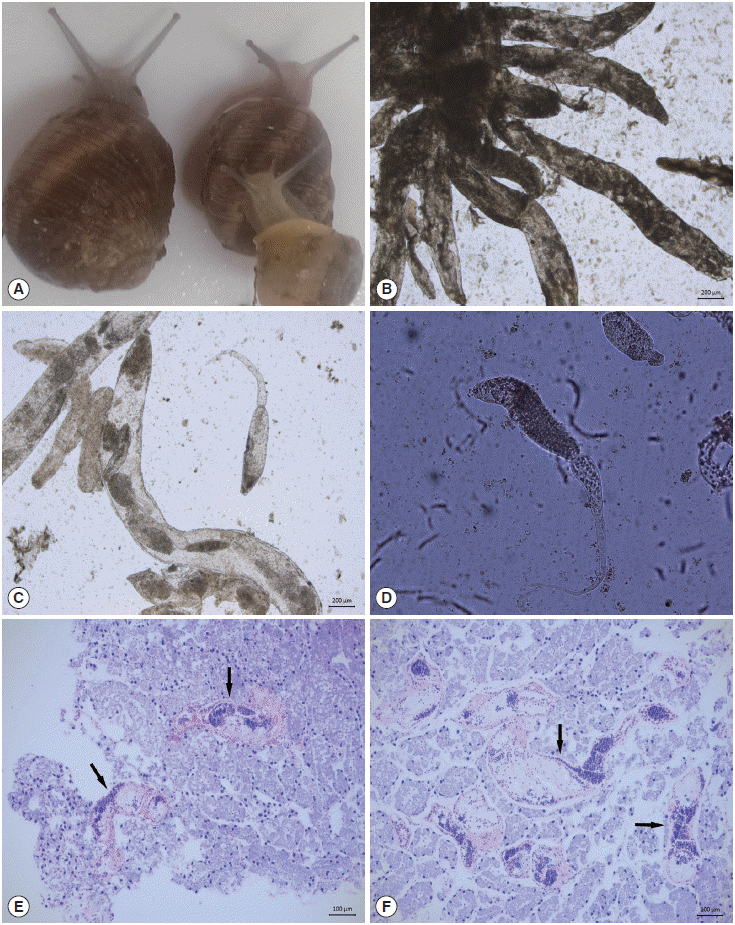
The intermediate host and larval stages of D. dendriticum. (A) Helix aspersa. (B) Second generation sporocysts (daughter sporocysts). (C) Sporocysts and cercariae. (D) Cercariae. (E) Section of digestive gland infected by cercariogenic sporocysts with the longitudinally sectioned cercariae (arrow). (F) Section of a digestive gland heavily infected by cercariogenic sporocysts (arrow).
The second generation sporocysts (daughter sporocysts) and cercariae of D. dendriticum were observed in the digestive gland and hepatopancreas of 5 (2.4%) of 211 H. aspersa snails. Among 101 collected snails in April showed that only 2 of them had sporocysts having immature cercariae (Fig. 1B), whereas among 110 collected snails in May, 3 snails had sporocysts having mature cercariae (Fig. 1C) and free cercariae (Fig. 1D). In infected H. aspersa snails, a great part of the digestive gland and hepatopancreas were extensively replaced by sporocysts, with the loss of normal tissue architecture in the areas directly affected by parasite (Fig. 1E, F). The branched sporocysts of a Brachylaima sp. were observed in the digestive gland of 4 (1.9%) snails. Only 1 snail had mature spocysts (Fig. 2A) in April; however, mature sporocysts and free cercariae (Fig. 2B) were observed in 3 snails in May. Highly branched sporocysts occupied a large part of the digestive gland in heavily infected snails. We also observed that some snails contained various stages of developmental forms of D. dendriticum and Brachylaima sp. A digenean trematode Brachylaima sp. was seen for the first time in Turkey.
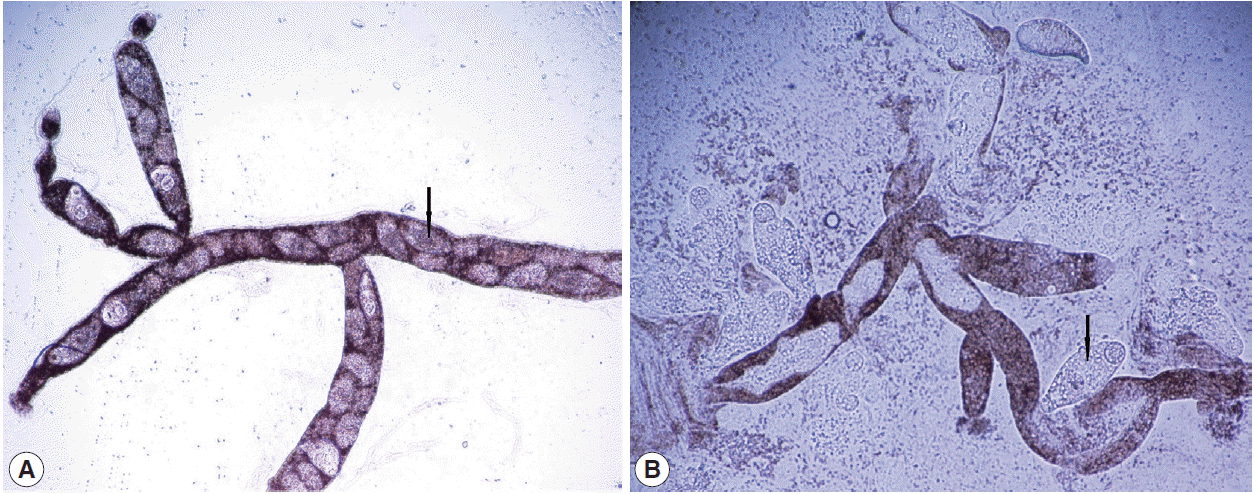
Larval stages of Brachylaima spp. (A) Highly branched cercariogenic sporocyst. (B) Sporocysts and cercariae (arrow).
In this study, the presence of larval stages of D. dendriticum and Brachylaima sp. in 211 H. aspersa snails was shown in the vicinity of Mersin District in the Mediterranean coast of Turkey and the prevalences were 2.4% and 1.9%, respectively. Previously, dicrocoeliid larval stages were reported in the same snail species with prevalence of 0.97% in the district of Izmir located in the Aegean coast of Turkey [7]. Our study is the second to investigate the larval stages of D. dendriticum in H. aspersa snails in Turkey. Gürelli et al. [29] reported dicrocoeliid larval stages in Helix lucorum snails with the prevalence of 27.6% in Kastamonu District in the Black Sea region, Turkey. The first intermediate host and prevalences for dicrocoeliid species reported at the Southern Part of Marmara region were as follows: Helicella itala 5.7%, Helicella candicans 4.3%, Helicopsis derbentina 4.0%, Monacha carthusiana 2.8%, Helicopsis krynickii 2.6%, Cernuella virgata 1.0%, Cochlicella acuta 0.4%, and Trochoidea pyramidata 0.2% [30]. Previous studies conducted in Turkey did not mention the presence of Brachylaima larval stages in H. aspersa and other snails. To our knowledge, this is the first that shows the presence of a Brachylaima sp. in Turkey. In Spain, H. aspersa snails were previously reported to be an intermediate host for Brachylaima aspersae [27, 28] and B. cribbi, a zoonotic species in south Australia [23].
H. aspersa snails collected in early April contained the second generation of sporocysts with germinal masses possessing the silhouette of cercariae or immature cercariae. At the late of April and May, collected snails were found to be infected with sporocysts having cercariae and free cercariae. Asexual development of D. dendriticum takes place within the snail, producing cercariae after 5-11 weeks [5,6]. Therefore, the snails may have been infected in autumn of the previous year or at the beginning of March in the current year.
It was concluded that H. aspersa acts as the first intermediate host of D. dendriticum and a Brachylaima sp. in Mersin District of Turkey, and cercariae become mature stage and leave the first intermediate host in May in this district. Moreover, in this study, some snails contained various stages of the developmental forms of D. dendriticum and Brachylaima sp. which are reported for the first time in Turkey.
Acknowledgements
The authors would like to thank Mr. Hayri Ertuğrul Cete who helped collecting live snails from pastures.
Notes
We declare that we have no conflicts of interest.