Tamoxifen Induces Apoptosis of Leishmania major Promastigotes in Vitro
Article information
Abstract
Tamoxifen is an antagonist of the estrogen receptor and currently used for the treatment of breast cancer. The current treatment of cutaneous leishmaniasis with pentavalent antimony compounds is not satisfactory. Therefore, in this study, due to its antileishmanial activity, effects of tamoxifen on the growth of promastigotes and amastigotes of Leishmania major Iranian strain were evaluated in vitro. Promastigotes and amastigotes were treated with different concentrations (1, 5, 10, 20, and 50 μg/ml) and time periods (24, 48, and 72 hr) of tamoxifen. After tamoxifen treatment, MTT assay (3-[4,5-dimethylthiazol-2-yl]-2,5 biphenyl tetrazolium bromide assay) was used to determine the percentage of live parasites and Graph Pad Prism software to calculate IC50. Flow cytometry was applied to investigate the induction of tamoxifen-induced apoptosis in promastigotes. The half maximal inhibitory concentration (IC50) of tamoxifen on promastigotes was 2.6 μg/ml after 24 hr treatment. Flow cytometry analysis showed that tamoxifen induced early and late apoptosis in Leishmania promastigotes. While after 48 hr in control group the apoptosis was 2.0%, the 50 µg/L concentration of tamoxifen increased it to 59.7%. Based on the in vitro antileishmanial effect, tamoxifen might be used for leishmaniasis treatment; however, further researches on in vivo effects of tamoxifen in animal models are needed.
INTRODUCTION
Cutaneous leishmaniasis (CL) is one of the most common parasitic diseases, which is transmitted by sand flies. It is one of the major public health and social problems in developing countries and throughout the world. According to World Health Organization, 1.5 million new infections are annually reported for this disease. Up to 90% of leishmaniasis cases occur in 7 countries; Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria. Based on geographical distribution, CL is divided into 2 groups, old world and new world CL. The disease of old world is caused by Leishmania major and Leishmania tropica [1-3].
CL is treated by different chemical drugs such as miltefosine, paromomycin, amphotericin B, and allopurinol [4]. Pentavalent antimonial compounds like pentostam and glucantime has been used to treat CL for the last 50 years [4,5]. However, the use of these compounds has some limitations such as long duration of treatment, high expenses of drugs, and methods of drug use which are intradermal and intramuscular injection. Beside these, lack of response to the treatment in 10-15% of cases and toxic effects on heart, liver, and kindneys are other possible side effects [4-6]. Currently, extensive research is being conducted wordwide to improve the methods of leishmaniasis treatment. Tamoxifen is a drug commonly used to treat breast cancer and has antagonistic effects with estrogen receptors on cell surface. Due to regulation effects on estrogen receptors, tamoxifen is used to improve ovulation in women and spermatogenesis in men as well. However, some of its biological effects, such as changes in cell calmodulin caspases and kinases, ceramid metabolism impairment, and prevention of acidification of intracellular organelles are independent from the drug´s ability to modulate estrogen mechanisms [7].
Studies on the mechanism of induction of apoptosis in cell lines without estrogen receptor have shown that tamoxifen increases activation of enzyme caspase-3 in a dose- and time-dependent manner. However, no significant changes in apoptotic proteins Bcl-2 and Bax level were observed [8]. The lethal effect of tamoxifen on Candida albicans, Coccidioides immitis, Leishmania braziliensis, and Leishmania chagasi has been shown previously [9-11]. Also a study showed that tamoxifen has a lethal effect on Leishmania as well [11]. In 2011, another study showed that tamoxifen could decrease the mean lesion diameter of Egyptian L. major compared to the control group in mice [7]. The present study was designed to investigate the effect of tamoxifen to induce apoptosis on Iranian strains of L. major.
MATERIALS AND METHODS
Parasite and culture
This study was carried out with Iranian L. major strain (MRHO/IR/75/ER). Leishmania promastigotes were cultured in RPMI-1640 medium with penicillin (100 units/ml), streptomycin (100 μg/ml), and 20% FBS (fetal bovine serum). A hundred µl of media containing 2×105 promastigotes were cultured in each well of 96-well plates and treated with 100 µl of different concentrations of tamoxifen (1, 5, 10, 20, and 50 µg/ml). Numbers of parasites were counted at 24, 48, and 72 hr of drug treatment. All the results are the average of triplicate experiments. Glucantime was used as positive control drug. IC50 of tamoxifen and glucantime were calculated by Graph-Pad Prism 5 software.
Determining the viability of promastigotes by MTT Assay
A hundred µl of culture media (RPMI-1640 with 20% FBS) containing 105 promastigotes were added to each well of 96-well plates. A hundred µl of different concentrations of tamoxifen or glucantime was added to designate wells. Two hundred µl of media were added to 3 wells as negative controls. After 24, 48, and 72 hr incubation, 20 µl of MTT reagent (5 mg/ml) was added to each well and incubated for 4 hr at 24˚C in dark. The plate was centrifuged at 3,000 rpm for 10 min. Then, the supernatant was removed from wells, and 100 µl DMSO (dimethyl sulfoxide) was added to each well. The optical density of the wells was read at 450 nm by an ELISA reader. The following formula was used to calculate the cell viability percent: Cell viability=[AT-AB]/[AC-AB] ×100, where AT is the optical density of wells with cells treated with the drug, AB is the optical density of blank wells, and AC is the optical density of control wells [12].
Evaluation of amastigote growth in mouse macrophages treated with tamoxifen
Macrophages (105/well) were cultured in 96-wells in 100 µl RPMI media and incubated at 37˚C with 5% CO2 for 24 hr. Promastigotes in stationary phase were added to each well at a parasite/macrophage ratio of 10:1, to contaminate macrophages. After 24 hr, in order to eliminate promastigotes which did not enter cells, the supernatant was discarded and new fresh medium was added. Then, macrophages containing parasite were affected with different doses of drugs. Numbers of amastigotes were counted in 100 macrophages, and the efficacy of different doses of the drug was calculated 24 hr after addition.
Analysis of apoptosis by flow cytometry
Annexin V-FITC Staining kit (Biovision, Palo Alto, California, USA) was used to assay tamoxifen-induced apoptosis in promastigotes. Parasites exposed to different concentrations of tamoxifen were collected after 24 or 48 hr and were centrifuged at 5,000 rpm for 5 min. Then, the supernatants were discarded, and 500 µl of binding buffer, 5 µl of annexin-V, and 5 µl of propidium iodide (50 µg/ml) were added, and the suspension was incubated for 5 min at room temperature in dark condition [13]. A flow cytometer (BD FACSCanto II, BD Bioscience, San Jose, California, USA) was used to detect apoptosis and FlowJo software (Tree Star, San Carlos, California, USA) was used to analyze the results.
Statistical analysis
The Kolmogorov-Smirnov normality and 2-way ANOVA tests were used to analyze and compare the results.
RESULTS
Percentage of live parasites
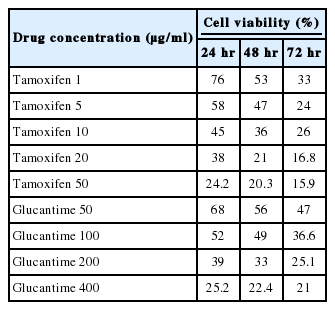
The percentages of live promastigotes and amastigotes at different concentrations of tamoxifen or glucantime after different incubation times are shown in Tables 1 and 2. All the results were statistically significant compared to the controls (P <0.05). These results were the average of at least 3 separate experiments. The difference between the mean values of treated cells with control cells was all statistically significant (P <0.05). IC50 of tamoxifen and glucantime was 2.6 and 82.9 µg/ml, respectively, after 24 hr.
MTT assay results
The percentages of viable cells were assayed at different concentrations of tamoxifen (1, 5, 10, 20, and 50 µg/ml) or glucantime (50, 100, 200, and 400 μg/ml) for 24, 48, and 72 hr. The results showed that tamoxifen decreased the viability of promastigotes in a concentration- and time-dependent manner (Table 3; Fig. 1).
Apoptosis of L. major promastigotes by flow cytometry
The percentages of early apoptotic cells (annexin positive), late apoptotic cells (annexin and propidium idoide positive) necrotic cells (propidium iodide positive) and living cells (annexin and propidium iodide negative) in 4 concentrations of tamoxifen were determined at 24 and 48 hr after culture. The highest (59.7%) and the lowest (16.0%) percentages of apoptosis in promastigotes after 48 hr of drug treatment occurred at 50 µg/ml and 1 µg/ml concentrations, respectively (Table 4; Fig. 2).

Percentage of programmed cell death (early and late apoptosis) at 24 hr and 48 hr after addition of various concentrations of tamoxifen
DISCUSSION
Pentavalent antimonial compounds are currently the first choice of treatment for CL [3,4]. However, the use of these compounds has different side effects. Many investigations have been done and is under way to improve leishmaniasis treatment using different chemicals or herbal medicine. For instance, Artemisia, Asafetida, and Aloe vera have been used in different studies for CL treatment [14-18]. Nanoparticles like ZnO can induce apoptosis in L. major in a dose- and time-dependent manner [12]. Some of anti-cancer drugs have been reported to have antileishmanial effects as well. Miltefosine is proven to induce apoptosis in Leishmania parasites [19].
Previous studies have shown that tamoxifen has antileishmanial effects [20]. Miguel et al. [11] investigated the efficacy of tamoxifen on cutaneous and visceral leishmaniasis by L. braziliensis and L. chagasi, respectively. Their results showed IC50 of tamoxifen against L. braziliensis and L. chagasi amastigotes was 1.9±0.2 and 2.4±0.3 mM, respectively. Lesion size in infected mice with L. amazonensis and parasite load in the liver and spleen of L. chagasi-infected hamsters were decreased [11]. Trinconi et al. [21] evaluated the interactions between tamoxifen and amphotericin B in L. amazonensis-infected BALB/c mice. Tamoxifen EC50 against promastigotes and amastigotes of L. amazonensis was 13.3 and 4.5 µM, respectively, and amphotericin B EC50 was determined to be 118.5 and 63.5 nM for promastigotes and amastigotes, respectively. Lesion size was reduced by 55% in the group assigned to low dose combined therapy [21]. Egyptian researchers evaluated the effect of tamoxifen on leishmanial lesions, and their results showed that tamoxifen was effective in inhibition and treatment of leishmanial leisons [7].
Our results showed that tamoxifen has growth inhibitory effects on promastigotes and also intra-macrophage amastigotes. These effects were dose and time-dependent. Therefore, increasing the dose and duration of the drug decreased the parasite growth rate. The parasite viability was also dose and time-dependent. So, by increasing the concentration and duration of the drug, the viability of promastigotes and intracellular amastigotes decreased. Our results showed that the highest growth inhibition was seen at 50 µg/ml of tamoxifen treatment for 48 hr. The flow cytometry results showed that tamoxifen-induced apoptosis was dose-dependent as well. Tamoxifen is a drug commonly used to treat breast cancer and has antagonistic effects with estrogen receptors on the cell surface [8]. In addition, tamoxifen increases the activation and production of certain enzymes involved in apoptosis, such as calmodulin, caspases, and cellular kinases. Disturbance in ceramid metabolism and blocking the acidification of intracellular organelles are the biological activity of tamoxifen [8,9].
The present research showed that tamoxifen can induce apoptosis in Leishmania promastigotes and amastigotes. However, the mechanisms of this pathway and proteins involved in induction of this cellular process are not clear.
Acknowledgements
The authors thank and appreciate all who have collaborated with this research. This study was funded supported by Deputy of Research, Kashan University of Medical Sciences (Kaums), Grant No. 91131.
Notes
We have no conflict of interest related to this work.