Isolation and Genotyping of Toxoplasma gondii Strains in Ovine Aborted Fetuses in Khorasan Razavi Province, Iran
Article information
Abstract
Toxoplasmosis is an important zoonotic disease that can cause abortion in humans and animals. The aim of this study was isolation and subsequent genotyping of Toxoplasma gondii isolates in ovine aborted fetuses. During 2012-2013, 39 ovine aborted fetuses were collected from sheep flocks in Khorasan Razavi Province, Iran. The brain samples were screened for detection of the parasite DNA by nested PCR. The positive brain samples were bioassayed in Webster Swiss mice. The serum samples of mice were examined for T. gondii antibodies by IFAT at 6 weeks post inoculation, and T. gondii cysts were searched in brain tissue samples of seropositive mice. The positive samples were genotyped by using a PCR-RLFP method. Subsequently, GRA6 sequences of isolates were analyzed using a phylogenetic method. The results revealed that T. gondii DNA was detected in 54% (20/37, 95% CI 38.4-69.0%) brain samples of ovine aborted fetuses. In bioassay of mice, only 2 samples were virulent and the mice were killed at 30 days post inoculation, while the others were non-virulent to mice. The size of cysts ranged 7-22 µm. Complete genotyping data for GRA6 locus were observed in 5 of the 20 samples. PCR-RLFP results and phylogenetic analysis revealed that all of the isolated samples were closely related to type I. For the first time, we could genotype and report T. gondii isolates from ovine aborted fetuses in Khorasan Razavi Province, Iran. The results indicate that the T. gondii isolates are genetically related to type I, although most of them were non-virulent for mice.
INTRODUCTION
Toxoplasma gondii is an intercallular protozoan that infects humans and animals. Sheep can be infected by ingesting oocysts excreted in cats’ feces and transplacental transfer [1]. Infection with T. gondii can lead to abortion, stillbirth, and birth of weak lamb in sheep flock [2,3]. Toxoplasma strains have been categorized into 3 clonal lineages (I, II, and III) according to virulence in outbreed mice. Previous studies have proved that the type I strains are lethal to mice, whereas experimental infection of mice with type II or III strains represented low virulence [4]. Recently, several molecular techniques such as multilocus enzyme electrophoresis [5], PCR-RFLP [6,7], microsatellite method [8,9], and multilocus DNA sequencing [10] have been used for T. gondii genotyping. In most genotyping studies, multiple genetic markers were analyzed by using multilocus PCR-RFLP and microsatellite methods [6,8], although these methods do not always provide satisfactory results due to insufficient amounts of extracted parasite DNA. Among different markers, GRA6 gene is a single copy gene and more polymorphic than the others markers and could clearly differentiate 3 different genotypes (I, II, and III) by using a single PCR reaction followed by single endonuclease (MseI) digestion [11].
Many studies have been done about genotyping of T. gondii in humans and animals around the world. The population structure of Toxoplasma has been analyzed with a variety of molecular probes. The results have shown that the number of T. gondii types is more than the classical types, and some strains have been categorized under atypical types [11,12]. Available data about T. gondii genotypes of ovine aborted fetuses is limited. So far, the type II, III, and atypical ones have been reported in aborted and healthy sheep from UK, Denmark, France, USA, Brazil, Italy, and Ethiopia [13-21].
In Iran, the overall prevalence of Toxoplasma infection was estimated to be 31% in sheep [22], and a few studies have been reported about genotyping of T. gondii in sheep. The type II and III have been identified in adult sheep using GRA6 locus along with 5 satellite markers [23]. The type I was found in ovine aborted fetuses using BI gene as a marker [24]. So far, Toxoplasma infection has been recognized as an important causative abortion in sheep flocks of Khorasan Razavi Province, Iran [25,26]. The aim of this study was to isolate and identify T. gondii genotypes in ovine aborted fetuses in the province.
MATERIALS AND METHODS
Study field
Khorasan Razavi Province is located in northeastern Iran (33˚30´-37˚41´ N; 56˚19´-61˚18´ E), with an area >127,000 km2. The northern part of the province is mountainous and has suitable conditions for agricultural activity and animal husbandry, while the southern part is mostly semi-desert and desert, with poor vegetation cover.
Sample collection
From 2012 to 2013, 39 aborted ovine fetuses were collected from different areas of the province. First, the fetuses and fetal membranes were grossly examined for any macroscopic lesions, and the age of aborted fetuses was also determined by crownrump length [27]. Then, the fetuses were necropsied to collect the brain tissue for molecular and bioassay examinations.
DNA extraction and PCR
Genomic DNA was extracted from samples using MBST Genomic DNA kit (Institute of Molecular and Biological Transmission Systems, Tehran, Iran) as per manufacturer’s recommendations. Subsequently, a nested PCR assay was carried out to detect the T. gondii B1 gene as previously described by Burg et al. [28].
Bioassay in mice
A 100 g of brains of positive aborted fetuses was homogenized in 500 ml of aqueous 0.85% NaCl solution (saline) with antibiotics (100 IU/ml penicillin and 745 IU/ml streptomycin) and then homogenized by electrical mixture. At the end, it was passed through 2 layers of gauze. After 3 hr incubation at room temperature, the samples were centrifuged at 1,500 g for 5 min, and 3 ml of the homogenate deposit was administrated subcutaneously to 3 Swiss Webster mice (1 ml per each mouse). At 6 weeks post inoculation, blood samples were taken from tail of mice, and 1:16 dilution of serum was tested to detect T. gondii antibodies by IFAT [29]. Seropositive mice were sacrificed at 42 days post infection by chloroform-inhalation. For detection of cysts, brain impression smears were prepared and microscopically examined. The inoculated mice were considered as infected when the mice had antibody titers ≥10 against Toxoplasma infection, or tissue cysts were found in the brain.
Genotyping of T. gondii
T. gondii DNA was extracted from the brain of infected mice, and genotyping was performed by PCR-RFLP with GRA6 marker as described previously [30]. Briefly, the PCR reaction was performed in a 25 µl reaction mixture containing 10 pmol of each primer (forward primer, 5´-GTAGCGTGCTTGTTGGCGAC-3´, and reverse primer, 5´-ACAAGACATAGAGTGCCCC-3´) and 10 µl of extracted DNA, 75 mM Tris-HCl (pH 8.5), 20 mM (NH4)2SO4, 1.5 mM MgCl2, 0.1% Tween 20, 0.2 mM dNTPs, 0.025 U/µl amplicon Taq DNA polymerase, inert red dye, and a stabilizer. The PCR conditions were 5 min at 95˚C followed by 35 cycles of 30 sec at 94˚C, 1 min at 60˚C, 2 min at 72˚C, and a final elongation of 72˚C for 7min. The PCR products were separated on a 1.5% agarose gel with 1× TAE buffer and visualized by staining with ethidium bromide. The GRA6 amplification product was exposed to MseI enzyme for digestion. Briefly, 15-ml of PCR product was digested using 1.5 U of MseI endonuclease and 2 U buffer R and incubated at 65˚C for 4 hr in accordance with the manufacturer’s protocol. The restriction fragments were separated by electrophoresis in 1.6% agarose gel followed by staining with ethidium bromide and visualization under UV. The cut position of MseI in GRA6 genes of types I, II, and III was 168 bp and 712 bp, 71 bp and 694 bp, and 71 bp, 168 bp, and 712 bp, respectively [30].
Gene sequencing and phylogeny
The PCR products purified and sequenced in the facilities of Bioneer Inc. (Seoul, Korea). The primers, which were previously used for PCR product of GRA6, were applied for the sequencing reactions. The nucleotide sequences were assembled and edited using CLC bio software. They were aligned with nucleotide sequences of GRA6 genes of different references T. gondii strains using ClustalW method. The phylogenetic analysis was carried out using neighbor-joining analysis with bootstrap values of 1,000 replicates (Programme Mega software version 6).
Ethics and animal experimentation
The mice were housed and maintained in the animal care facility at Ferdowsi University of Mashhad, Iran. All animal experiments were performed in strict accordance with the guidelines approved by the Animal Ethics Committee of our faculty.
RESULTS
The brain samples of 37 ovine aborted fetuses were examined by semi-nested-PCR, and T. gondii DNA was detected in 54% of the brain samples (Fig. 1). The positive samples were bioassayed in mice during the experimental study, and 15% of mice (9/60, 95% CI 8.2-26.2%) died between 18-30 days post infection. The sera of live animals were examined at 6 weeks post infection using IFAT, and 55% of mice (33/60, 95% CI 42.5-66.9%) resulted positive for the T. gondii antibody assay. Tissue cysts of T. gondii were identified in brain tissues of inoculated mice by direct microscopy. The size of cysts ranged 7-22 µm.

PCR amplification products of Toxoplasma gondii B1 in aborted fetuses brain samples Lane M, molecular weight marker (between 1,000 and 50 bp); 1, positive control (Toxoplasma tachyzoites); 2, negative control (H2O instead of DNA); 3-6, positive samples.
The brain samples of mice were analyzed by GRA6 PCR method. Totally, complete genotyping data for GRA6 locus were observed just in 5 isolates (Fig. 2). In order to detect the genotype of Toxoplasma samples, GRA6 products were digested with MseI restriction enzyme, and the type of samples was determined by digestion patterns. All positive DNA fragments had 2 cut sites for MseI restriction enzyme, as the digestion pattern of type I (Fig. 3). Detailed information concerning the samples has been summarized in Table 1. The amplified GRA6 genes of the 6 isolates were sequenced and deposited in GenBank (NCBI) under the accession nos. of KM372587 (TgShir 1) and KM372588 (TgShir 2). The nucleotide sequences of GRA6 gene from isolates were aligned with nucleotide sequences of T. gondii (Table 2). The sequenced PCR products were found to have 99% homology with the analysis. The sequence homologies in the neighbor-joining analysis of strains isolated from ovine aborted fetuses and references strains of T. gondii showed that all of the isolates were clustered in type I strain (Table 2; Fig. 4).

PCR amplification products of Toxoplasma gondii GRA6 in mice brain samples. Lane M, molecular weight marker (between 1,000 and 100 bp); 1-2, positive control (Toxoplasma tachyzoites); 3, negative control (H2O instead of DNA); 4-5, positive samples.
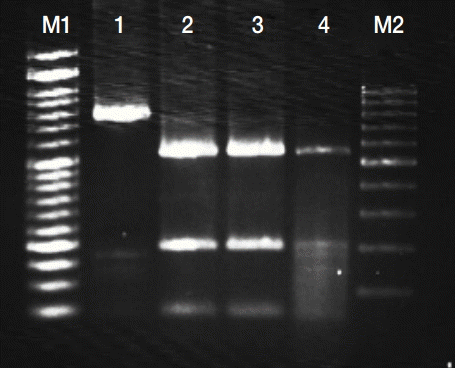
PCR-RFLP analysis of GRA6 gene coding region with MseI endonuclease. Lanes M1 and M2 are DNA size markers (between 1,000 and 100 bp). Lane 1, undigested PCR products of GRA6 gene; 2-4, digested PCR products of GRA6 gene for Type 1.

Results of Bioassay and genotyping of Toxoplasma gondii isolated from ovine aborted fetuses which were T. gondii DNA positive cases by nested PCR

Comparison of nucleotide sequences of GRA6 gene coding of T. gondii isolates from Mashhad area, Iran with reference strains
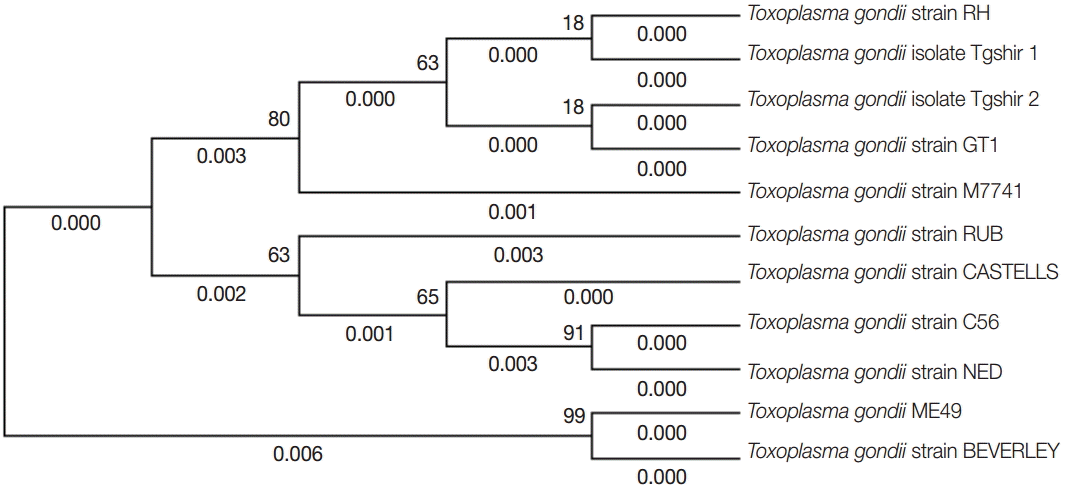
Phylogenetic analysis of representative Iran isolates (Tgshir1,Tgshir2) and reference strains; Strain RH (Type I), GTI (Type I), ME 49 (type II), M7741 (type III), NED (type III), Beverley (type III), RUB (Atypical), C56 (Atypical), and CASTELLE (Atypical). The tree was constructed using the neighbor-joining method after bootstrapping with 1,000 repetitions.
DISCUSSION
In this study, T. gondii DNA was detected in 54% of brain samples of aborted fetuses in sheep. T. gondii infection has been detected in sheep aborted fetuses to range between 13.5% to 61% in different areas of Iran by PCR assays [24,26,31]. The bioassayed results showed that 15% of inoculated mice died before 30 days, and the other inoculated mice (85%) developed antibodies against T. gondii and survived until sacrificed time, thus indicating that the majority of isolates belong to avirulent strain. In the previous study, an avirulent strain was isolated from aborted ovine fetuses in Iran by bioassay method [25]. The results of other studies indicated that most of the T. gondii isolates from sheep were non-virulent for mice [15,16,18,21]. In the present study, the tissue cysts with small size (7-22 µm) were observed in the brain of inoculated mice at 6 weeks post infection. The size of Toxoplasma tissue cysts is depending on the age of the cyst. The young tissue cysts may be small and contain only 2 bradyzoites while older ones may contain hundreds of organisms; tissue cysts in the brain are often spherical and reach a diameter of 70 mm [32,33].
The PCR-RFLP based on GRA6 revealed that all isolates in this study from ovine aborted fetuses belong to type I clonal lineage. This result is in agreement with a previous study that type I was also detected in 12 sheep aborted fetuses in Iran using B1 gene as a target sequence for PCR-RLFP [24]. In another study, type II and III were reported in 4 isolates from adult sheep according to microsatellite and GRA6 gene sequence analysis in Iran [23]. In other countries, Owen and Trees [13] detected type II using PCR assays based on the SAG2 locus in the placentas of 13 aborted sheep from UK. Likewise, Jungersen et al. [14] showed that 11 (6 from aborted lambs, 5 from healthy sheep) isolates of T. gondii from Denmark were type II. Similarly, another study using the SAG2 locus and 5 satellite markers (TUB2, TgM-A, W35, B17, and B18) proved that all 8 T. gondii isolates derived from adult sheep in France were clonal type II [15].
Interestingly, during a study on 10 PCR-RFLP markers, 47 of 57 T. gondii isolates in USA were type II or III, while the remaining isolates were reported as atypical [16]. Moreover, they could isolate an atypical strain by using same markers in a flock of purebred Suffolk ewes on a farm in Texas [17]. Recently, a study has been performed on aborted ovine fetuses on Sardinia Island, Italy. They indicated that all isolates were type II by using 11 markers [20]. According PCR-RLFP of the GRA6 gene, the type I, II, and III of Toxoplasma were reported from pigs in Japan [34], the type II and III from humans, chicken, duck, and cat in Iran [23] and type II and III from humans in Cyprus [35].
In the present study, the GRA6 sequencing and phylogenetic analysis confirmed that our 2 isolates were closely related to type I (RH and GTI strains). Although the coding region of the GRA6 locus is a considerably polymorphic target and more variable than the other markers examined, it could clearly differentiate the 3 different genotypes (I, II, and III) by using a single PCR reaction [30]. However, recent studies have shown that the high genetic diversity among T. gondii isolates in livestock and typing of T. gondii isolates using 1 molecular marker is not so reliable to detect atypical strains from type III and I [7,9,10]. On the other hand, in the classic genotyping of T. gondii, type I isolates were virulent with high mortality in mice [1], while in the present study, only 10% of T. gondii isolates were virulent for mice. Therefore, it seems that the report of type I from ovine aborted fetuses is questionable and need more investigation.
In conclusion, T. gondii DNA was detected in the brain samples of ovine aborted fetuses. Our results showed that T. gondii isolates were closely related to the type I, whereas the results pathogenically indicated significant differences in mice. We suggest examining more molecular markers for identification of unknown strains to obtain more credible results. Prospectively, determining the predominant type of T. gondii in endemic areas such as Khorasan Province could pave the way for control, prevention, and development of an effective vaccine.
Acknowledgements
We are very grateful to Drs. Zahra Naseri, H. Eshrati, and G. A. Azari for their technical assistance. This study was supported by a grant no. VPRTFM-3.27748 from the Vice President Research and Technology of Ferdowsi University of Mashhad, Iran.
Notes
The authors declare that they have no conflict of interest.