Animal Models for Echinostoma malayanum Infection: Worm Recovery and Some Pathology
Article information
Abstract
Echinostomes are intestinal trematodes that infect a wide range of vertebrate hosts, including humans, in their adult stage and also parasitize numerous invertebrate and cold-blooded vertebrate hosts in their larval stages. The purpose of this study was to compare Echinostoma malayanum parasite growth, including worm recovery, body size of adult worms, eggs per worm, eggs per gram of feces, and pathological changes in the small intestine of experimental animals. In this study, 6-8-week-old male hamsters, rats, mice, and gerbils were infected with echinostome metacercariae and then sacrificed at day 60 post-infection. The small intestine and feces of each infected animal were collected and then processed for analysis. The results showed that worm recovery, eggs per worm, and eggs per gram of feces from all infected hamsters were higher compared with infected rats and mice. However, in infected gerbils, no parasites were observed in the small intestine, and there were no parasite eggs in the feces. The volume of eggs per gram of feces and eggs per worm were related to parasite size. The results of histopathological changes in the small intestine of infected groups showed abnormal villi and goblet cells, as evidenced by short villi and an increase in the number and size of goblet cells compared with the normal control group.
INTRODUCTION
Echinostomes, intestinal flukes, have been found in epidemic proportions in Southeast Asia [1]. Previous studies have shown that echinostome infection is one of the most common parasitic infections that adversely affect the health of the population in this region [2]. The prevalence of infection, based on finding echinostome eggs in the stool or the presence of worms at endoscopic autopsy [3], suggests efficient transmission in some parts of the world [4]. Intestinal parasitic infections are typically caused by dietary habits, such as eating raw/undercooked freshwater snails [5]. After eating infected snails or other intermediate hosts, the body releases secretions known as antigens, which stimulate the immune system, as evidenced by the inflammatory cell response to antigens of the parasite in animal models [6].
In human cases, several reports have addressed the signs and symptoms of light-to-moderate infections, e.g., anemia, headache, dizziness, slight stomachache, gastric pain, and loose stools [7,8]. Endoscopic observation has found that echinostomes damage the small intestinal mucosa and cause extensive intestinal and duodenal lesions [9] and inflammation [10]. Thus far, ethical limitations have restricted pathological studies in humans; therefore, animal models have been used to explore the biology, life cycle, and pathology of echinostomes, including host-parasite interactions. However, there are more than 20 species of echinostomes worldwide [5]. Although there have been reports in animal models for some echinostomes, each species has certain differences in biology and life cycle, as described above. Therefore, the present study investigated animal models for Echinostoma malayanum, focusing on its morphology, biology, and pathology.
MATERIALS AND METHODS
Preparation of metacercariae
Freshwater snails, Indoplanorbis exustus, were collected in an endemic region in Khon Kaen Province, Thailand. Snails were pounded coarsely, added to a blender containing pepsin solution in a ratio of 4:6 by volume, and mixed for 3-5 min. After digesting in a shaking water bath at 37˚C for 1 hr, the subshells were passed through a series of filters (1,000, 300, and 106 μm, respectively) using saline (0.85% NaCl) as a diluent. The sediment that settled in a sedimentation jar was examined for the presence of metacercariae of echinostomes; these were collected under a stereomicroscope.
Animal infection and specimen collection
Syrian hamsters, Wistar rats, outbred ICR mice, and gerbils (6-8-week-old males) were used for a study of E. malayanum in animal models. Each animal in the 4 assigned groups was infected with 50 metacercariae and other 4 uninfected groups. At day 60 post-infection, all animals were sacrificed by anesthetizing with diethyl ether, weighed, and then the small intestine (containing adult worms) was removed. Fecal pellets were collected from the rectum. The experimental protocol was approved by the Animal Ethics Committee of Khon Kaen University (no. AEKKU35/2557).
Eggs per gram of feces
To determine the indirect reproductive organ development, feces from each infected hamster, rat, and mouse were collected for detection of eggs per gram of feces. No eggs were found in infected gerbils. Modified formalin technique was performed for the quantitative echinostome egg count. One pellet of feces from the rectum was weighed, fixed, and mixed thoroughly with 1,000 µl of 10% formalin. The solution (200 µl) was smeared with 1% iodine solution on a glass slide, and the number of echinostome eggs was counted under a stereomicroscope. The procedure was repeated 3 times and the results averaged. The number of eggs per gram was calculated as follows:
Eggs per worm
Five worms from infected hamsters, rats, and mice were individually crushed with 70% ethyl alcohol by a mortar. No worms were found in infected gerbils. Each solution was added to a 15 ml centrifuge tube, and the volume adjusted with 70% ethyl alcohol to 10 ml. Fifty µl of each solution was smeared with 1% iodine solution on a glass slide, and the number of echinostome eggs was counted under a stereomicroscope. The procedure was repeated 3 times, and the results were averaged. The number of eggs per worm was calculated as follows:
Worm recovery
To compare the worm recovery from infected hamsters, rats, and mice, the worms from the small intestine of each animal were collected in saline solution and then counted.
Body size of echinostomes
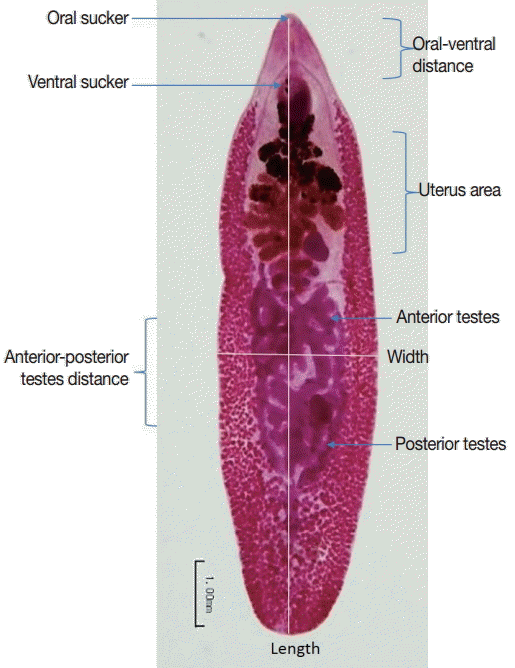
To determine the parasite development in infected hamsters, rats, and mice, 5 adult worms were randomly chosen for measurement of body size using carmine staining. Adult worms were fixed in 70% ethyl alcohol, stained with carmine for 2 hr, de-stained with 1% acid–alcohol, and then dehydrated in a series of ethyl alcohol (70%, 80%, 90%, 95%, and absolute, respectively) for 24 hr. Finally, dehydrated flukes were mounted with permount solution on glass slides and measured the body size, width, length, oral-ventral suckers distrance, and anterior-posterior testes distance using image analysis as shown in Fig. 1.
Histopathological study
To determine the histopathological changes post-echinostome-infection in hamsters, rats, and mice, the small intestines were fixed in 10% formalin, washed with PBS for 2 hr, and prepared for sectioning. The specimens were processed by an automatic machine as follows: dehydrated in an alcohol series; 70% ethyl alcohol for 1 hr (2 times), 80% ethyl alcohol for 1 hr (2 times), 95% ethyl alcohol for 1 hr (3 times), absolute ethanol for 1 hr (3 times), followed by clearing in xylene for 1 hr (2 times). They were then paraffinized in an incubator at 60˚C with paraffin solution for 1.5 hr (1 time) and 2 hr (1 time), respectively. Tissues embedded in paraffin blocks were cut with a microtome into tissue sections (4-5 µm) which were coated on slides. The tissue section slides were placed in an incubator at 60˚C for 24 hr and then kept at room temperature.
Small intestine tissue sections were deparaffinized with xylene for 3 min (3 times) and then rehydrated in an alcohol series and then stained with hematoxylin and eosin (H-E). Finally, the tissue sections were dehydrated in a series of ethyl alcohol; 70% ethyl alcohol for 3 min (3 times), 95% ethyl alcohol for 3 min (3 times), and absolute ethanol for 3 min (3 times), followed by clearing in xylene for 3 min (3 times), and then mounted on slides with permount.
Small intestine tissue sections stained with H-E were counted for the number of goblet cells per high-power field (HPF) (× 400), studied over 10 selected HPFs. Results were presented as the mean number of cells per HPF and SD.
RESULTS
Worm recovery
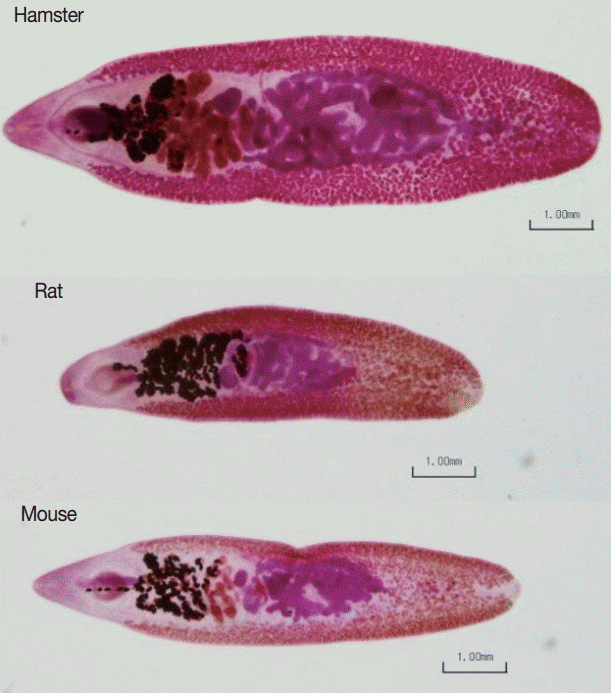
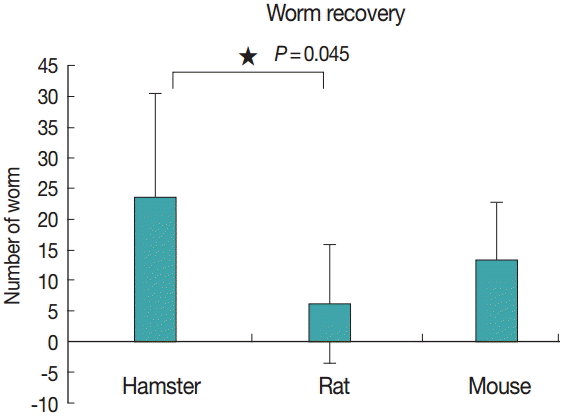
Figs. 2 and 3 show representative worm samples and the average worm recovery for each animal model, respectively. No worms were observed in gerbils. The average worm recovery from hamsters (48%) was higher than that from mice (26%) and rats (12%). The worm recovery from hamsters was significantly different from rats (P =0.045), but not significantly different from mice (P >0.05). All worms completely revealed their reproductive organs, ovary, eggs in uterus, 2 tandem testes, and vitelline gland.
Body size of echinostomes
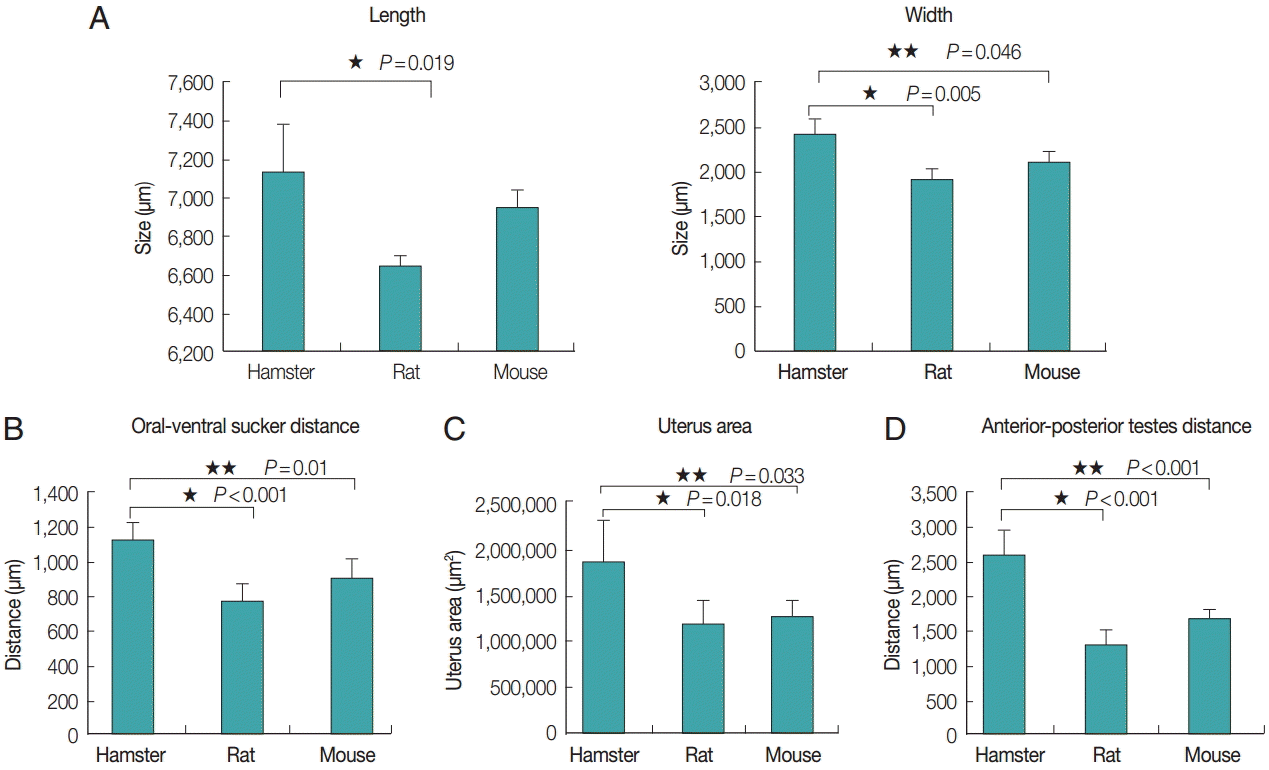
The average length and width of E. malayanum adults recovered from hamsters, rats, and mice were 7,133 ×2,355, 6,646×1,859, and 6,938×2,051 µm, respectively (Fig. 4A). The average length of E. malayanum from hamsters was longer than those from rats (P =0.019) and mice (P >0.05). The average width of E. malayanum from hamsters was wider than those from rats (P =0.005) and mice (P =0.046). The oral-ventral sucker distrance (Fig. 4B), uterus area (Fig. 4C), and antero-posterior testes distrance (Fig. 4D) of E. malayanum recovered from hamsters were significantly (P <0.05) larger than those observed in rats and mice.
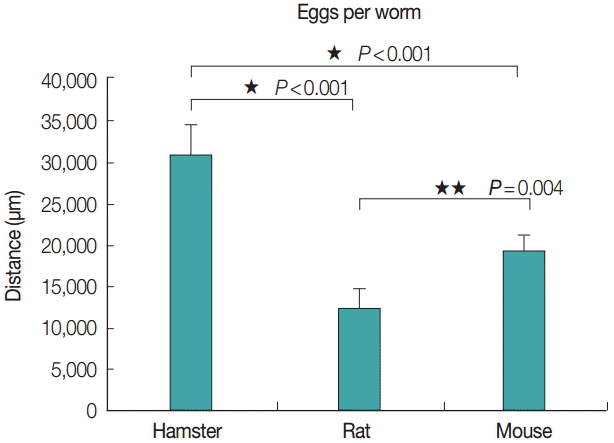
Eggs per worm
The average eggs per worm from each animal model was correlated with the adult worm size, as evidenced by the eggs per worm from hamsters, rats, and mice: 30,560±3,575, 12,240±2,331, and 19,180±1,791, respectively (Fig. 5). The number of eggs per worm from hamsters was higher than that from rats and mice, with statistically significant differences (P <0.001, P =0.004).
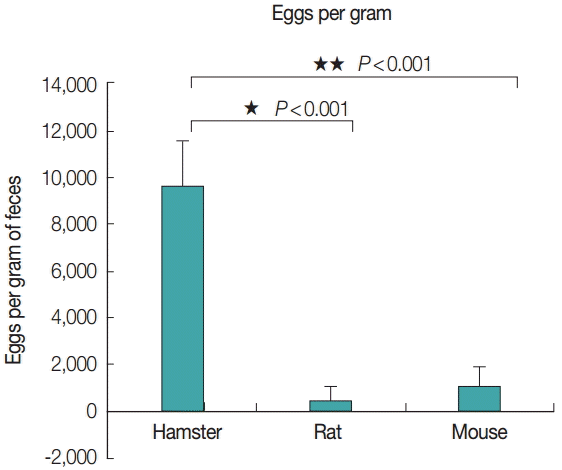
Eggs per gram of feces
The average eggs per gram of feces from each animal model was also correlated with the adult size, as evidenced by the eggs per gram of feces from hamsters, rats, and mice; 9,675, 508, and 1,108, respectively (Fig. 6). The number of eggs per gram of feces from hamsters was significantly (P <0.001) higher than that from rats and mice, with a statistically significant difference.
Intestinal histopathological changes
The intestinal villi of all hamsters, rats, and mice in infected groups were shorter compared with those of normal control groups. Goblet cell hyperplasia was evidenced by an increase in number and size which was significantly increased in hamsters (P=0.004), rats (P=0.002), and mice (P=0.001) (Figs. 7, 8).
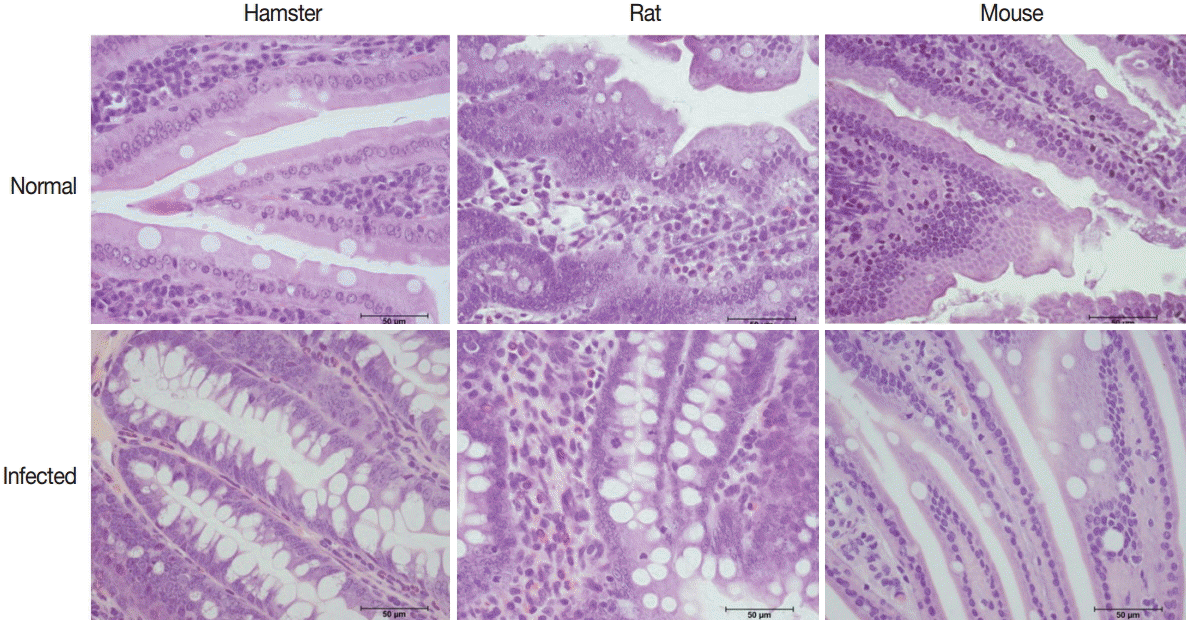
Representative histopathological changes in the small intestine of normal and E. malayanum-infected hamsters, rats, and mice.
DISCUSSION
Several years ago, there were reports on E. malayanum development in mice, but the behavior of this parasite in other animals has yet to be explored. The present study is the first report on hamster, rat, mouse, and gerbil animal models for E. malayanum. We found that E. malayanum could infect hamsters, rats, and mice, but not gerbils. A 100% infectivity rate was found in both hamsters and mice, while a 60% infectivity rate was observed in rats. The worm recovery showed the protective immunity of each animal model. The largest size of adult worms was observed in hamsters, correlated with the high number of eggs per worm and eggs per gram of feces. The small intestine pathology of infected hamsters was more severe compared to that of rats and mice, as evidenced by greater local tissue damage and inflammatory responses, goblet cell hyperplasia, and disruption of villi.
E. malayanum infection in an animal model was reported in Swiss albino mice [11,12]. The infectivity rate was 100%, which agreed to the findings in our present study. Worm recoveries were as high as 73.6% at the end of the experiment, which was much higher than in our present study (26%). This disparity may be due to using a different type of mouse model, which may have different host immunity [13]. Srisawangwong et al. [11] reported that the length of the worms rapidly increased during the first 3 weeks (from 1.43 mm at week 1 to 5.12 mm at week 3), and then increased slowly thereafter, reaching a peak at week 11 (7.73 mm). The width increased slowly and appeared to remain stable from week 5 onwards. In the present study, the size of the worms was smaller compared with the results of the above-mentioned report [11]. Moreover, our study showed different size of adult worms in different host models which was different from a previous report which showed the same size of E. revolutum adults recovered from different hosts, i.e., hamsters and domestic chicks [14].
The worms were mature and began to produce eggs after 2 weeks post-infection. Eggs, detected by modified formalin-ethyl acetate concentration technique, were found in feces after 2 weeks. Egg output increased at week 3 and remained stable until the end of the experiment at 12 weeks post-infection [11]. The present study is the first report that shows the fecundity of E. malayanum adults through eggs per worm in each animal model. The Syrian hamster was found to be a highly compatible host and the Wistar rat a less compatible host for E. malayanum. This was similar to a previous report [15] which found that the golden hamster was a highly compatible host, and the Wistar rat a less compatible host for E. caproni. Moreover, the number of worms from hamsters was higher than those observed in rats, similar to the present result.
In the small intestine of rats, mice and hamsters, hyperplasia of goblet cells is the mechanism for the expulsion of intestinal helminths [6]. Goblet cells are highly selective and specific effector cells of the host defense mechanism [16]. A high response was observed in the hamster model, but difference was noted from E. caproni-infected hamsters [15]. In chronic E. caproni infection, shortened villi were observed in all infected rats, mice, and hamsters [17,18]. However, in this study, hamsters were shown to be more susceptible to E. malayanum than rats and mice. Gerbils were not susceptible to E. malayanum infection. Worm recovery was correlated with greater mucosal damage, inflammation, and goblet cell hyperplasia. These results may be beneficial for future animal studies on various aspects of echinostome infection.
Acknowledgements
This study was supported by grants from Research Affairs, Faculty of Medicine, Khon Kaen University (no. IN59128), and the Thailand Research Fund (no. RTA5880001 and RDG5620033).
Notes
We have no conflict of interest related to this work.