In Vitro Scolicidal Effects of Salvadora persica Root Extract against Protoscolices of Echinococcus granulosus
Article information
Abstract
It has been known that Arak, Salvadora persica, has a number of medicinal properties. We tried to investigate in vitro scolicidal effect of root extracts of this plant against protoscolices from hydatid cysts of Echinococcus granulosus. Protoscolices were aseptically collected from sheep livers containing hydatid cysts. S. persica root extract was used in 10, 30, and 50 mg/ml concentration for 10, 20, and 30 min. The viability of protoscolices was ascertained by 0.1% eosin staining. Scolicidal activity of S. persica extract at a concentration of 10 mg/ml was 36.3%, 50.3%, and 70.8% after 10, 20, and 30 min of exposure, respectively. The scolicidal effect of this extract at a concentration of 30 mg/ml was 52.9%, 86.7%, and 100% after 10, 20, and 30 min of exposure, respectively. S. persica extract at a concentration of 50 mg/ml, meanwhile, killed 81.4%, 100%, and 100% of protoscolices after 10, 20, and 30 min, respectively. Also, the cytotoxic potential of S. persica was assessed on human liver cells (HepG2) using trypan blue exclusion test. No cytotoxic effect was observed on HepG2 cell line. The present study confirmed for the first time that the ethanolic extract of S. persica has high scolicidal power in vitro. However, in vivo effect of this material remains to be studied for treatment of echinococcosis in humans and herbivorous animals.
INTRODUCTION
Echinococcosis or hydatidosis is a serious zoonotic infection with a worldwide distribution, caused by larval stages of cestodes belonging to the genus Echinococcus [1]. Infection with Echinococcus granulosus, the causative agent of cystic echinococcosis, is found on all continents, with the highest prevalence in parts of Eurasia (especially Mediterranean countries, the Russian Federation and adjacent independent states, and China), north and east Africa, Australia, and South America [2]. Hydatid cysts of E. granulosus develop in internal organs (mainly the liver and lungs) of humans and other intermediate hosts as unilocular fluid-filled bladders [3]. Although the initial phase of the infection is always asymptomatic for many years or even permanently [2]. Without efficient treatment, the continuous development of the cyst will ultimately result in organ malfunction and even death in many cases [4].
Currently, there are 3 treatment choices for hydatidosis: surgery, which remains the foremost effective treatment, percutaneous aspiration, and medicinal treatment [5]. Surgery is still the preferred method of treatment but one of the major surgical complications of hydatidosis is the recurrence after operation for primary hydatid disease [6]. The spillage of protoscolices-rich fluid during surgery is a major cause of recurrence and multiple secondary hydatidosis [7]. The use of effective scolicidal agents, while avoiding spilling the cyst contents is an integral part of the surgical technique for this intervention, helping to reduce the risk of spilling viable protoscolices [8]. Many scolicidal agents have been used to inactivate the content of cysts, but most are not safe due to their side effects such as sclerosane cholangititis (biliary tract fibrosis), liver necrosis, and methemoglobinemia [9]. The development of safe and effective new scolicidal agents, especially from natural sources, is therefore of great interest [10].
Arak (Salvadora persica) is one of the most commonly used medicinal plants for treatment of rheumatism, leprosy, gonorrhea, ulcers, scurvy, tumors, and dental diseases [11]. Arak has been used for centuries as a natural toothbrush (as Mesiwak or Siwak), especially in Muslim countries as a sunnah of the prophet, while its fibrous branches have been promoted by the World Health Organization for use in oral hygiene [12].
No laboratory studies have documented the effect of S. persica on protoscolices of hydatid cysts. Therefore, the present study was conducted to investigate the in vitro scolicidal effect of root extracts of this plant against protoscolices of E. granulosus at different exposure times. Also, the cytotoxic effect of S. persica extracts was evaluated using trypan blue exclusion test on human liver cells (HepG2).
MATERIALS AND METHODS
Collection of protoscolices and viability test
Hydatid cysts of E. granulosus naturally infected in the liver of sheep were obtained from a slaughterhouse in Riyadh city, Saudi Arabia. The intact cysts were immediately placed in an ice-box and transported to the Parasitology Laboratory, Zoology Department, College of Science, King Saud University, Riyadh, Saudi Arabia. Hydatid fluid along with protoscolices was collected according to Smyth and Barret [13]. The collected fluid with protoscolices was transferred into glass cylinders and left to set for 30 min to allow the protoscolices to settle to the bottom of the cylinders. The supernatant was then discarded, and the yielded protoscolices were washed 3 times in normal saline.
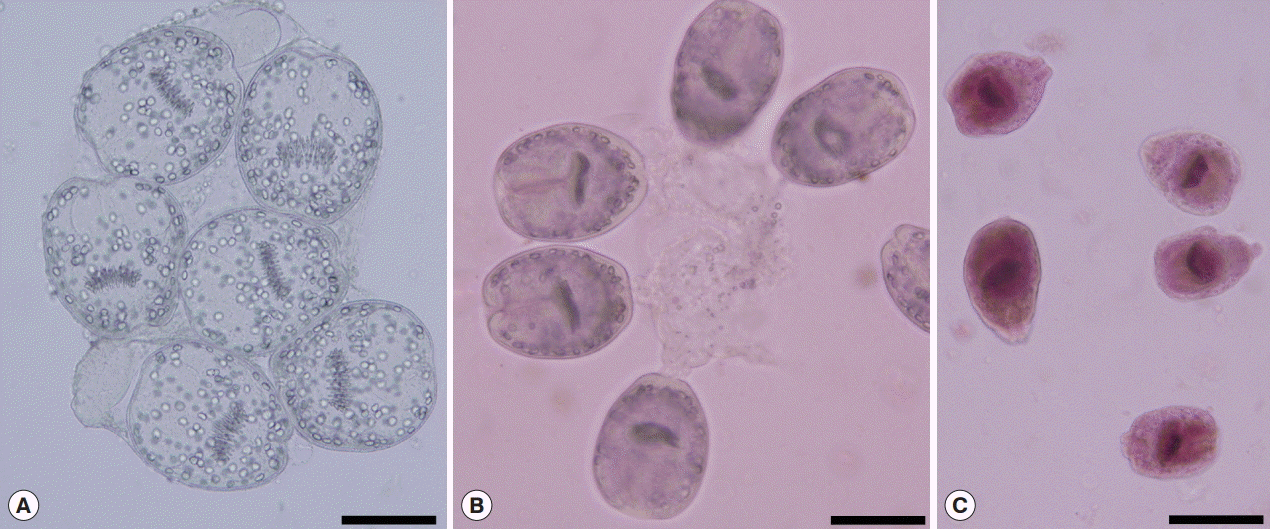
The viability of protoscolices (Fig. 1A) was confirmed from their motility characteristics under light microscopy. The viability was also assessed using 0.1% aqueous solution of eosin stain (1 g of eosin powder in 1,000 ml distilled water). Five min after exposure to the stain, unstained protoscolices were considered as viable (Fig. 1B), while stained protoscolices were considered as non-viable (Fig. 1C) [14]. When 95% or more viable protoscolices were present in the sediments, the sample was considered to be suitable for further experiments, and these were transferred into a dark container containing normal saline and stored at 4˚C until use.
Preparation of S. persica extract
S. persica L. (Salvadoraceae) roots (Arak) were collected from Jizan, Saudi Arabia. The identification was confirmed by a local expert at the herbarium of the Botany and Microbiology Department, College of Science, King Saud University. Roots were cut into small pieces, and ground into a fine powder using an electrical blender. About 100 g of root powder was extracted using 400 ml of 70% ethanol. The mixture was agitated for 30 min [15], and then maintained at rest for 24 hr. The resulting extract was filtered using a sterile filter paper, and then the solvent was completely removed using a rotary evaporator.
In vitro scolicidal tests
We tested 3 concentrations (10, 30, and 50 mg/ml) of S. persica extract for 10, 15, and 30 min. To prepare these concentrations, respectively, 0.1, 0.3, and 0.5 g of dried extract was dissolved in 10 ml of distilled water. Then, 2.5 ml of each concentration was placed in a test tube to which about 10,000 washed protoscolices was added. The contents of the tube were mixed gently, and the tube was then incubated at 37˚C for 10, 15, or 30 min. At the end of each incubation period, the supernatant was discarded carefully to avoid disturbing the settled protoscolices. One ml of 0.1% eosin stain was then added to the remaining settled protoscolices and mixed gently. After 5 min, the upper portion of the solution was again discarded. The remaining pellets of protoscolices were smeared on a glass slide, covered with a cover glass, and examined microscopically for viability. The percentages of dead protoscolices were determined by counting at least 450 protoscolices. Non-treated protoscolices in normal saline were considered as a control group. Each experiment was performed in triplicate.
Cytotoxicity assessment
Cell culture
Human live cell line (HepG2) that used in the experiment was obtained from American Type Culture Collection (ATCC) (Manassas, Virginia, USA). HepG2 cells were cultured in DMEM, supplemented with 10% FBS, 0.2% sodium bicarbonate, and antibiotic/antimycotic solution (1 ml/100 ml of medium). Cells were grown in 5% CO2 at 37˚C in high humid atmosphere.
Trypan blue dye exclusion test
The test was based on the principles that live cells with an intact cellular membrane that exclude the trypan blue dye while dead cells uptake the dye and stained blue. The test was performed following the protocol of Siddiqui et al. [16]. In brief, HepG2 cells were exposed to various concentrations (10, 30, 50 mg/ml) of ethanol extracts of S. persica for different time intervals (10, 20, and 30 min). After the respective exposure, cell suspensions were aspirated, centrifuged at 2,000 rpm for 3 min and washed with sterile PBS twice immediately after the incubation. The cells were stained with trypan blue (0.4% solution at a ratio of 1:5, dye: cell suspension) and counted using a hemocytometer. The counting for live (unstained) and dead (blue stained) cells was made at 100× magnification under a phase-contrast inverted microscope. The untreated cells were also run simultaneously under the identical conditions and served as the control. Each experiment was performed in triplicate.
Statistical analysis
Differences between the test and control groups were analyzed with chi-square test using a statistical package program (Sigma Plot version 11.0). P-values less than 0.01 were considered to be significant.
RESULTS
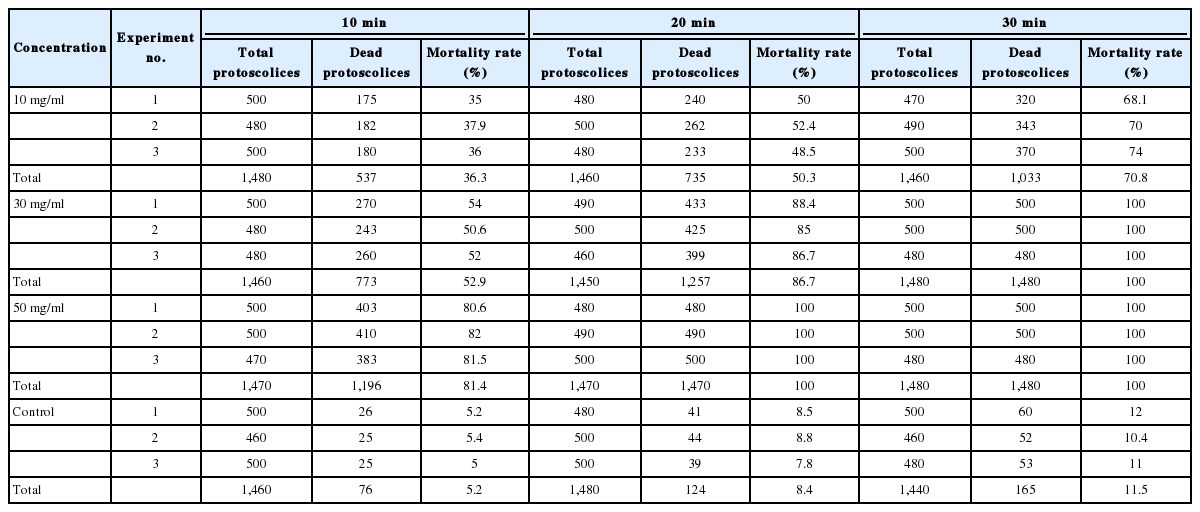
The results of the present experiment revealed highly significant (P<0.001) scolicidal effects against protoscolices of E. granulosus for all of the various concentrations of S. persica ethanolic extract, compared to the control results, both within the same period of time and different periods as shown in Table 1. While the maximum death rate in the control group was 11.5%, scolicidal activity of S. persica extract at a concentration of 10 mg/ml was 36.3%, 50.3%, and 70.8% after application for 10, 20, and 30 min, respectively. The scolicidal effect of the extract at a concentration of 30 mg/ml was 52.9%, 86.7%, and 100% after application for 10, 20, and 30 min, respectively. S. persica extract at a concentration of 50 mg/ml, meanwhile, killed 81.4%, 100%, and 100% of protoscolices after 10, 20, and 30 min, respectively.
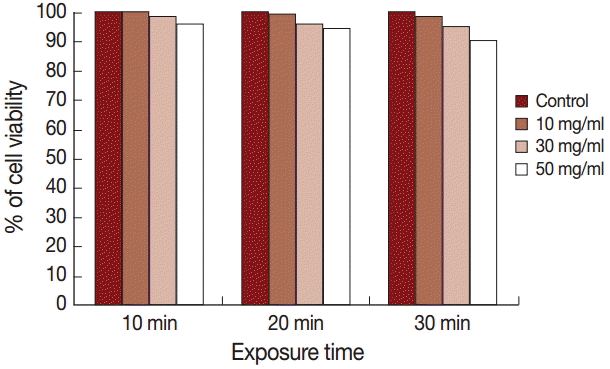
The results of the cytotoxicity assay using trypan blue exclusion test are shown in Fig. 2. The cell viability was 100%, 99%, and 98% after exposure to 10 mg/ml for 10, 20, and 30 min, respectively, while it was 98%, 96%, and 95% after exposure to 30 mg/ml for the same time intervals. Application of 50 mg/ml extract for 10, 20, and 30 min showed cell viabilities of 96%, 94%, and 90%, respectively.
DISCUSSION
Hydatid disease (hydatidosis) is a parasitic disease that remains a clinical problem worldwide, especially in areas where animal husbandry and subsistence farming form an integral part of community life [17]. Seventy-five percent of all hydatid cysts are found in the liver [18], and these are usually managed either by surgery or medicinal treatment [19]. Surgery is associated with considerable mortality, morbidity, and recurrence rate. It is also expensive, and, clearly, needs expertise [20]. Chemotherapy can be used as treatment for patients considered not fit for surgery, or as an adjuvant to surgical treatment preoperatively or postoperatively, or both [19]. Various degrees of success have been claimed for many drugs that are now being used in hydatidosis treatment [21]. However, the metabolites of these drugs, including albendazole, albendazole sulfoxide, benzimidazole, and mebendazole, are potentially toxic in some subjects and are associated with severe hepatobiliary complications [22]. Natural scolicidal agents offer a safe alternative with no adverse associated effects [9].
Researchers around the world have begun to explore the real effect of many plants traditionally used as medicines which have not hitherto been validated by rigorous scientific experimentation. In this context, recently, many plants have been screened for their in vitro scolicidal activities, including Ocimum bacilicum and Allium cepa [6], Sambucus ebulus [9], Trachyspermum ammi [10], Allium sativum [14], Zingiber officinale [23], and Zataria multiflora [24].
With respect to S. persica, Reuben et al. [25] confirmed very high anthelmintic effects (86.7% and 98.9%) for aqueous extracts of S. persica at concentrations of 25 mg/ml and 50 mg/ml, respectively, against strongylid nematodes. These results urged us to test the in vitro scolicidal effect of ethanolic extract of S. persica. It was shown in this study that the ethanolic extract of S. persica exhibited the strongest scolicidal effect (100% and 100%) after 20 min at a concentration of 50 mg/ml and after 30 min at a concentration of 30 mg/ml. The ethanolic extract of S. persica is reported to contain a number of important phytoconstituents, such as indole alkaloids (e.g., salvadoricine), flavonoids (e.g., quercitin), the sulphur-containing compound tropaedoin, triterpenes, phytosterols, and isothiocyanates (e.g., benzyl isothiocyanate) [26]. Sofrata et al. [26] proved that S. persica root sticks consist of more than 98% benzyl isothiocyanate, while another report has confirmed that benzyl isothiocyanate is the predominant or sole anthelmintic agent in papaya seed extracts against Caenorhabditis elegans [27]. Our results showed a high scolicidal effect of S. persica root extracts against protoscolices of E. granulosus, which might be due to the presence of anthelmintic isothiocyanates. The present study proved that the ethanolic extract of S. persica have no cytotoxic effect on HepG2 cells. Similarly, previous studies have found the ethanol extract of this plant to be completely devoid of cytotoxic effects on HGFs and on L929 consistently in 3 cytotoxic assays [28]. Studies have also shown that the extract is safe in respect to liver and kidney functions and hematological parameters of rats [29]. Also, Ahmed et al. [30] confirmed that the ethanolic extract of S. persica did not show any untoward effect up to a dose of 5,000 mg/kg body weight and did not cause death in any tested albino mice.
In conclusion, the root ethanolic extract of S. persica is a safe and potent protoscolicide with the potential to be used in hydatid cyst treatment and pre-surgery to prevent secondary cyst recurrence. However, in vivo efficacy of this extract remains to be explored, and further studies are necessary to identify and isolate the active compounds. This is the first report on the scolicidal activity of S. persica.
Acknowledgements
We extend our appreciation to the Dean of Scientific Research, King Saud University, for funding the work through the research group project no. RG-004.
Notes
The authors declare that the present study followed the ethical standards and the ethical rules applicable for this journal and no conflict of interest.