Abstract
The WD40-repeat proteins serve as a platform coordinating partner proteins and are involved in a range of regulatory cellular functions. A WD40-repeat protein (CsWD1) of Clonorchis sinensis previously cloned is expressed stage-specifically in the tegumental syncytium of C. sinensis metacercariae. In the present study, interacting proteins with the CsWD1 protein was purified by immunoprecipitation and 2 dimension gel electrophoresis from the C. sinensis metacercaria soluble extract, and tryptic peptides were analyzed by LC/ESI-MS. Putative partner proteins were annotated to be actin-2, glyceraldehyde-3-phosphate dehydrogenase, and hypothetical and unmanned proteins. The CsWD1 protein was predicted to contain 3 conserved actin-interacting residues on its functional surface. With these results, the CsWD1 protein is suggested to be an actin-interacting protein of C. sinensis.
-
Key words: Clonorchis sinensis, WD40-repeat protein, actin
The WD40-repeat proteins occur ubiquitously in cells of all eukaryotes and are involved in a wide range of cell functions, such as vesicular trafficking, signal transduction, RNA transcription and processing, and regulation of cytoskeleton assembly and cell division (
Ono, 2003). Proteins of the WD40-repeat family have 4-10 repeat units containing 44-60 amino acids. Core region of the repeat unit ends with the dipeptides Gly-His (GH) and Trp-Asp (WD) at the N- and C-termini (
Smith et al., 1999). The WD40-repeat units consisting of 4 anti-parallel β-sheets fold into the β-blades and then into a circular β-propeller. The β-propeller structure of WD40-repeat proteins is stable and rigid, not relaxed upon binding interacting partner proteins. It serves as a platform coordinating simultaneous and/or sequential multi-protein interactions by holding them in a close proximity. The WD40-repeat domain has been identified not having catalytic activity.
The actin-interacting proteins (Aip1) are members of the WD40-repeat family of proteins and have been identified, with crystallographic structure, to contain as many as 14 WD40-repeats forming 2 β-propeller domains (
Voegtli et al., 2003;
Mohri et al., 2004). In the presence of actin depolymerizing factor (ADF)/cofilin-bound actin filaments, the Aip1 enhances actin filament fragmentation by capping ends of served filaments. The Aip1 is involved in actin-dependent cellular processes, such as restricting the localization of cofilin to cortical patches of yeasts (
Ida and Yaha, 1999), organizing actin filaments in muscle cells of
C. elegans (
Mohri and Ono, 2003), and cytoskeleton reorganization during regeneration of chicken auditory epithelium (
Oh et al., 2002). The Aip1 has the ability to enhance turnover of ADF/cofilin-bound actin filaments. However, the Aip1 itself exerts very weak activity on actin filament dynamics.
The WD40-repeat cDNA (CsWD1) was cloned and its expression was characterized from
C. sinensis metacercariae. The CsWD1 protein comprising of 7 WD40-repeats was predicted to fold into anti-parallel β-sheets then into a β-propeller. The CsWD1 protein was expressed stage-specifically in the tegumental syncytium of the metacercariae, but not in the adults (
Cho et al., 2007). cDNAs of partner proteins interacting with the CsWD1 protein were cloned using yeast two-hybrid screening, and extended with EST sequences retrieved from the
C. sinensis EST pools. The annotated partner proteins were 4 signal proteins, 3 transporters, 1 protease, and 1 muscle protein (
Kim et al., 2007).
In life cycle of
C. sinensis, the metacercariae are encysted in the flesh of the second intermediate host, freshwater fishes, and run basal metabolism. Upon taken up by the final mammalian hosts, the metacercariae grow up rapidly to adult flukes. During growth and development, the body of metacercariae becomes enlarged and the genital primordia differentiate into reproductive organs in middle region of the body of
C. sinensis adults (
Rim, 2005). In the metacercariae, the CsWD1 protein is expected to function as a platform holding proteins close together and to render protein-protein interactions. The present study was performed to clone out partner proteins and to get more information on molecular function of the WD40-repeat protein in the
C. sinensis metacercariae.
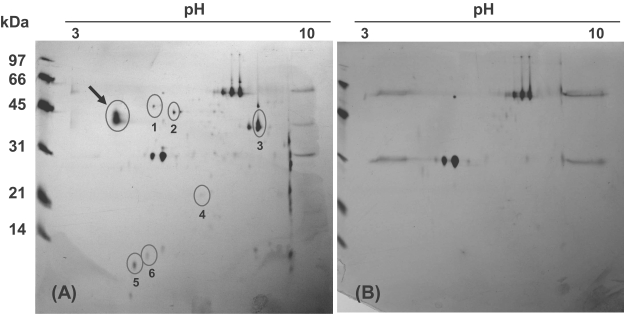
To identify interacting partner proteins, immunoprecipitation experiments were performed using anti-CsWD1 monoclonal antibody and C. sinensis metacercaria soluble extract. The soluble extract was prepared by grinding and sonicating the metacercariae with 2 volumes of 1× MB buffer (10 mM K-HEPES, 20 mM KCl, 1 mM EGTA, 3 mM MgCl2, 1 mM DTT) containing protease inhibitor cocktail, and by spinning them at 4℃. To remove non-specific proteins binding to the Protein A, 50 µl of soluble extract of C. sinensis metacercaiae was mixed with 50 µl of Protein A resin in 500 µl of PBS and stirred at 4℃ for 2 hr. After spinning, the pellet of the Protein A resin was taken out from the extract mix. This metacercarial extract, 100 µl, was mixed with 100 µl of anti-CsWD1 monoclonal antibody concentrated using Protein A column. After adding Protein A resin, the mix was stirred further for 2 hr and washed with PBS. The CsWD1-partner protein complex was eluted with 2-DE rehydration buffer. IPG strips rehydrated with the prepared samples were electrofocused using Ettan IPGphor Isoelectric Focusing System (Amersham Biosciences Inc., Uppsala, Sweden) then loaded on 2-dimension SDS-polyacrylamide gel. Spots of the putative partner proteins and CsWD1 were excised from the silver-stained gel. The proteins were digested with trypsin and eluted from the gel slices. Tryptic peptides of the partner protein were analyzed by LC/ESI-MS using Q-TOF2 micromass spectrometer (Micromass, Manchester, UK) at Korea Basic Science Institute (Daejeon, Korea). Control samples were run in parallel without the metacercarial soluble extract.
Two dimensional gel electrophoresis of the CsWD1 complex revealed 6 putative partner proteins (
Figs. 1A, 1B). Spots 1 and 2 produced tryptic peptide sequences (GYSFTTTAER, EITALAPSTMK, EITALAPSTMK + Oxidation (M), QEYDESGPGIVHR + Pyro-glu, QEYDESGPGIVHR, SYELPDGQVITIGNER, VAPEEHPVLLTEAPLNPK, DLYANTVLSGGTTMFPGIADR) matching actin-2 (P53471) of
Schistosoma mansoni, and those of spots 3 and 5 matched glyceraldehyde-3-phosphate dehydrogenase (P20287) of
S. mansoni, and other spots were identified to unnamed or hypothetical proteins of mice.
The Aip1 protein is a member of the WD40-repeat family proteins with two 7-bladed β-propeller domains (
Ono, 2003), and the N-terminal propeller of the Aip1 proteins plays a primary role in the interaction with F-actins. Three actin-interacting amino acid residues were identified, each on the surface of the β-propeller of Aip1 in
Saccharomyces cerevisiae and
Caenorhabditis elegans. Among the amino acid residues, 2 phenylalanine residues each in blade 4 and the loop connecting blades 4 and 5 were conserved between the 2 species (
Mohri et al., 2004;
Okada et al., 2006). D177 and F212 of the CsWD1 were found at equivalent positions in the
C. elegans Aip1, and F212 and D217 of the CsWD1 appeared at corresponding sites of the
S. cerevisiae Aip1. E126 of the
C. elegans Aip1, residing in blade 3, is spatially close to D168 and F192, which are also conserved in the CsWD1. The E126 residue is crucial for filament disassembly but not F-actin-binding. An E126A mutant showed binding activity to F-actin (
Mohri et al., 2004). The
Arabidopsis thaliana Aip1, in which E126 was substituted with glycine at the equivalent position, binds F-actin in pollen grains (
Allwood et al., 2002). In the CsWD1, an alanine residue resides at the equivalent position. These results suggest that the CsWD1 has 3 conserved actin-interacting residues on its putative functional surface. Based on the above evidence, the CsWD1 is proposed to be an actin-interacting protein of
C. sinensis.
Notes
-
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST; R01-2002-000-00261-0).
References
Fig. 1Purification of partner proteins of the CsWD1 protein. (A) Putative interacting proteins in a 2-dimensional gel. Arrow indicates the native CsWD1 protein which was proven by peptide sequencing as described in the text. Circle locates putative partner protein. (B) Control group. Spots of upper and lower rows are heavy and light chains of immunoglobulin G, respectively.

Citations
Citations to this article as recorded by

- Clonorchis sinensis and clonorchiasis, an update
Sung-Tae Hong, Yueyi Fang
Parasitology International.2012; 61(1): 17. CrossRef