Gynaecotyla squatarolae (Digenea: Microphallidae) from rats experimentally infected with metacercariae from the shore crab, Macrophthalmus dilatatus
Article information
Abstract
Metacercariae of Gynaecotyla squatarolae (Digenea: Microphallidae) were discovered from the shore crab, Macrophthalmus dilatatus, purchased at a market in a coastal town of Taean-Eup, Chungcheongnam-do, Republic of Korea. Their adult flukes were confirmed by experimental infection of rats. The metacercariae of G. squatarolae were elliptical (391.1 x 362.5 µm), and the excysted metacercariae had progenetic genital organs, including the ovary and testes. To obtain adult flukes, 6 Sprague-Dawley rats were fed 500 metacercariae each, and killed at days 2, 4, and 6 post-infection. The adult flukes were identified as G. squatarolae (Yamaguti, 1934) Yamaguti, 1939, based on morphological characters, including 2 ventral suckers (1 large and 1 small), a large genital atrium equipped with the cirrus and the metraterm, separated male and female genital pores, a transversely long cirrus pouch, and extensive vitelline follicles. In the present study, it has been first proven that the shore crab M. dilatatus is a second intermediate host for G. squatarolae in the Republic of Korea.
INTRODUCTION
Adult flukes of the family Microphallidae Travassos, 1920 occur as intestinal parasites of vertebrates and take arthropods as their second intermediate hosts (Yamaguti, 1958; Schell, 1985). More than 17 genera are known in this family, which include Gynaecotyla, Maritrema, Spelotrema, Pseudospelotrema, Carneophallus, Levinseniella, Microphallus, and Microphalloides (Yamaguti, 1958; Schell, 1985). In the genus Gynaecotyla (Yamaguti, 1934) Yamaguti, 1939, 4 valid species have been known; G. squatarolae (type), G. adunca, G. simillima, and G. riggini (Dery, 1958). Species of Gynaecotyla have common morphological characters, including 2 ventral suckers, separate male and female genital pores, a well-developed genital atrium, and a large transverse cirrus pouch anterior to the ventral sucker (Dery, 1958; Schell, 1985).
The second intermediate host of the Microphallidae is known to be crustaceans (Schell, 1985). Stunkard (1968) described massive infection of the horseshoe crab, Limulus polyphemus, by the metacercariae of Microphallus limuli. Hunter (1952) found the metacercariae of G. adunca encysted in the fiddler crab, Uca pugilator. In the Republic of Korea, the marsh crab, Helice tridens tridens, and several other species of crabs were found infected with the metacercariae of Microphalloides japonicus (Seo et al., 1964). Metacercariae of a Levinseniella species were reported from the brackish water crab, Macrophthalmus japonicus, in the Republic of Korea (Choi et al., 1985).
Gynaecotyla squatarolae was first discovered in the small intestines of birds, Squatarola squatarola hypomelaena and Erolia alpina sakhalina, in Japan (Yamaguti, 1934, 1939), and then from experimentally infected rats, rabbits, chicken, and quail (Otagaki, 1958). Metacercariae of G. squatarolae were discovered from the brackish water crabs, i.e., Macrophthalmus dilatatus and M. japonicus in Japan (Yamaguti, 1934; Otagaki, 1958), and in M. japonicus in the Republic of Korea (Sohn, 1994). In the present study, we detected microphallid metacercariae in the crab, M. dilatatus, caught from a western coastal area, and identified the adult worm as G. squatarolae by experimental infection to rats.
MATERIALS AND METHODS
Collection of the metacercariae
In March 2007, 2 species of brackish water crabs, M. dilatatus (Fig. 1) and M. japonicus, were purchased from a local market in Taean-Eup, Chungcheongnam-do, Republic of Korea. The hepatopancreas of the crabs was separated, ground in a mortar with a pestle, and filtered through a series of nets. Microphallid metacercariae, 106 to 500 µm in size, were collected from the sediments using a stereomicroscope and washed several times with phosphate buffered saline (PBS). The collected metacercariae were used for experimental infection of rats, and some of them were excysted under a cover slip applying slight pressure. The excysted metacercariae were fixed in 10% neutral formalin for microscopic examinations.

The crab host, a metacercaria and adults of Gynaecotyla squatarolae. Fig. 1. Dorsal (left) and ventral (right) views of the shore crab, Macrophthalmus dilatatus, the second intermediate host. Fig. 2. A metacercaria from the crab, encysted. Bar = 120 µm. Fig. 3. An excysted metacercaria, showing the oral, ventral suckers, ovary, testes, cirrus sac, genital atrium, and vitelline follicles. Bar = 50 µm. Fig. 4. A 2-day-old juvenile worm recovered from an experimental rat, stained with Semichon's acetocarmine. Two ceca (C), an ovary (OV), 2 ventral suckers (VS), genital atrium (GA), testes (T), vitellaria (VT), and cirrus pouch with seminal vesicle (SV) are easily recognized. Bar = 60 µm. Fig. 5. A 6-day-old adult fluke recovered from a rat, stained with Semichon's acetocarmine. Numerous eggs are seen in the uterine loop, and the size of testes remarkably enlarged. Bar = 70 µm.
Experimental infection to rats
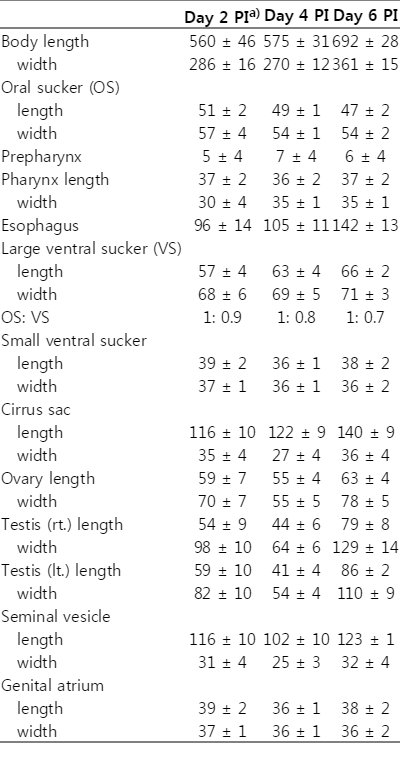
In order to obtain adult flukes, 6 Sprague-Dawley rats were experimentally fed 500 metacercariae each. They were sacrificed by cervical dislocation on days 2, 4, and 6 post-infection (PI). The small intestine was resected, divided into 3 portions, and opened longitudinally along the mesenteric border in PBS. The adult flukes were collected from the intestinal content using a stereomicroscope, fixed with 10% neutral formalin under a cover slip pressure, and measured after Semichon's acetocarmine staining. Measurements were done on 10 fixed specimens and given in µm with the mean followed by SD. To observe the eggs, the feces of the rats were examined by the formalinether sedimentation technique.
RESULTS
Infection status of crabs with the metacercariae
The brackish water crabs, which were purchased in a market in Taean-Eup, Chungcheongnam-do, consisted of 2 species, i.e. M. dilatatus and M. japonicus. They were different in color and in the morphology of the back. Both species were examined for the presence of metacercariae, and only M. dilatatus was proved to have the metacercariae of G. squatarolae. The mean number of metacercariae per crab was 4.3 (range; 1-11, no. of crabs examined; 850), and they were mainly located in the hepatopancreas of the crab. The metacercarial density increased according to the size of the crab (data not shown).
Recovery rate of adult flukes
The average worm recovery rate (WRR) of G. squatarolae adult flukes from rats was 9.4% through days 2-6 PI (Table 1). The WRR was the highest at day 6 PI (13.0%), and the lowest at day 4 PI (3.3%), but statistical difference was not noted. At day 2 PI, 42.7% of the adult flukes were recovered from the middle part of the small intestine, but, at days 4-6 PI, most worms were harvested from the posterior part of the small intestine; 71.7% at day 4 PI and 89.0% at day 6 PI.
Brief morphology of Gynaecotyla squatarolae (Yamaguti, 1934) Yamaguti, 1939
Metacercariae (Figs. 2-3): Encysted metacercariae, 391 x 363 (av.), elliptical with a thin wall (Fig. 2), with a small oral sucker and 2 ventral suckers, i.e., larger and smaller ones. Vitelline follicles 2 groups, lobulated, incorporating the excretory bladder between them. Excysted matecercariae, 480 x 270 (av.), and elongated (Fig. 3). Oral sucker subterminal, prepharynx long, and esophagus moderately long. Ceca widely divergent. Larger ventral sucker located at midline of the worm, posterior to the cirrus pouch. Smaller ventral sucker slightly postero-dextral to larger ventral sucker. Cirrus pouch lying transversely anterior to larger ventral sucker, and ending as genital atrium. Ovary between larger acetabulum and left cecal arch. Testes lying beneath each cecal arch, and larger than ovary. Genital atrium antero-dextral to smaller ventral sucker. Vitelline follicles grouped behind each testis, between them located excretory bladder.
Adults (Figs. 4-5): Body ovoid (Fig. 4), with varying size from 561 x 286 to 692 x 361 depending on age of infection (Table 2). General morphology common with excysted metacercariae with some differences. Oral sucker subterminal, prepharynx short, and esophagus moderately long. Ceca widely divergent and short, ending near level of testes. Larger ventral sucker at midline of the worm, equatorial or slightly post-equatorial, posterior to cirrus pouch. Smaller ventral sucker slightly dextral to larger ventral sucker. Cirrus pouch lying transversely anterior to two ventral suckers, containing voluminous seminal vesicle, ejaculatory duct and prostatic gland, and ending in genital atrium medially. Cirrus muscular, lying in genital atrium, composed of 2 spatulated and chitinized projections. Ovary between larger acetabulum and left cecal arch. Seminal receptacle and Mehlis' gland present. Testes larger than ovary, and lying beneath each cecal arch. Genital atrium antero-dextral to smaller ventral sucker. Vitelline follicles extensive, with more than 10 follicles on each side, locating posterior to testes. Uterine coils runs through posterior fields of body, with metraterm ends in genital atrium on median dorsal surface, with distal end reinforced by a heavy cuticular earlobe-shaped plate armed with about 15-20 tiny surface spines. Intrauterine eggs appearing at day 2 PI, occupying half of hindbody at day 4 PI and all available space of hindbody at day 6 PI (Fig. 5). Eggs 21 x 17 (av.), operculated, elliptical, brownish, and thick-shelled.
DISCUSSION
In the present study, the shore crab, M. dilatatus, has been proved to be a second intermediate host for G. squatarolae. Therefore, the second intermediate host for G. squatarolae in the Republic of Korea includes 2 species of brackish water crabs, M. japonicus (Sohn, 1994) and M. dilatatus (this study). The metacercariae were found usually in the hepatopancreas of the crab. This was the same as in another report that the metacercariae of Microphallus primas were located in the hepatopancreas of the crab host (Saville and Irwin, 1991).
The worms recovered in the present study were identified as a species of Gynaecotyla. First, the presence of 2 ventral suckers was one of the most important clues (Dery, 1958; Schell, 1985). Second, the genital atrium equipped with separated male and female genital organs (Hunter, 1952) was another generic characteristic. The cirrus pouch does not contain the cirrus, though having the seminal vesicle, ejaculatory duct, and prostatic gland; the cirrus is present in the enlarged genital atrium (Yamaguti, 1958; Dery, 1958). Two groups of vitelline follicles, located posterior to each testis, are another important characteristic of Gynaecotyla (Dery, 1958; Yamaguti, 1958).
Among the species of Gynaecotyla, G. adunca (Linton, 1905) is most similar to G. squatarolae (Yamaguti, 1934). However, G. squatarolae has more than 10 vitelline follicles on each side, whereas G. adunca has less than 10 follicles on each side (Dery, 1958). G. squatarolae differs from G. riggini Dery, 1958 in that the former has an ovary in the left side of the worm, whereas the latter has an ovary in the dextral position (Dery, 1958). Because of close similarities, G. nassicola (Cable and Hunnien, 1938) and G. jägerskiöldi (Travassos, 1920) had been synonymized with G. adunca (Dery, 1958). The major difference between G. simillima (Travassos, 1921) and G. squatarolae is the comparative size of 2 ventral suckers (Dery, 1958); the 2 suckers do not differ by more than 0.02 mm in diameter for G. squatarolae, but differ more than 0.02 mm for G. simillima (Dery, 1958). In our specimens, the smaller ventral sucker was smaller than the larger ventral sucker by 0.02-0.03 mm depending on age of worms. However, we assigned our specimens as G. squatarolae considering the geographical close proximity between Korea and Japan, where G. squatarolae was first discovered. G. simillima was originally described from an avian species, Nyctanassa violacea, in Brazil (Yamaguti, 1958). However, synonymy between G. squatarolae and G. simillima should be further elucidated.
The metacercariae of G. squatarolae contained sexual organs, such as the ovary, testes, and the cirrus pouch. In this regard, Caveny and Etges (1971) reported that microphallid metacercariae undergo extensive organogenesis, at times to the point of progenetic egg production in arthropod intermediate hosts. Thus, in the case of Microphallus opacus, the probability of completing its life history without involvement of a vertebrate host was suggested (Caveny and Etges, 1971). According to them, the discovery of adult M. opacus from mammalian hosts may be regarded as cases of an incidental parasitism rather than an obligate parasitism. In the present study, the metacercariae of G. squatarolae quickly excysted after extracting from the crab; hence, the necessity for a definitive host might not be very high like other trematode families. Similarly, the definitive host range of microphallid flukes may be wider than can be considered.
The natural definitive host for Gynaecotyla species is, in most cases, birds (Yamaguti, 1958). For example, adult specimens of G. riggini were recovered from a ruddy turnstone, Arenaria interpres morinella, from Florida, USA (Dery, 1958). The herring gull was proved to be heavily infected with G. adunca (Hunter, 1952). G. squatarolae was described from 2 avian species, S. squatarola hypomelaena and E. alpina sakhalina (Yamaguti, 1934). Rankin (1940) used young gulls as an experimental definitive host for G. nassicola. However, in the present study, we used rats as an experimental definitive host for G. squatarolae, and succeeded in obtaining adult flukes, as was done by a previous worker (Otagaki, 1958). By this, we speculate that the host range of G. squatarolae in nature may be wide. Efforts to search for natural definitive hosts are required.
Since crabs preserved in soy sauce or chilly sauce are favored by the Korean people, human infections with G. squatarolae might be existing. Therefore, epidemiological investigations should be done on the people living in coastal areas producing shore crabs. Considering that the eggs of G. squatarolae are very small in size, infected persons, if any, could be missed in screening fecal examinations.