Abstract
A paleoparasitological study was carried out on 2 lead coffins recovered from the Roman site of Jaunay-Clan (near Poitiers, France). For the first time, this particular type of burial gave positive parasitological results, and eggs of the whipworm Trichuris trichiura were identified in 1 individual. In the present case, thanatomorphose associated with funerary practices may explain the scarcity of the recovered eggs. However, human whipworm has now been observed in 9 individuals dated to the Roman period. The very high frequency of Trichuris sp. eggs in Roman archaeological sites (up to 80%) suggests that fecal peril, hygiene, and waste management were problematic during this period. Finally, due to the fact that very few analyses have been conducted on human bodies dated to the Roman period, more analyses must be performed in the future to provide further information about diseases in the Roman world.
-
Key words: Trichuris trichiura, trichuriasis, paleoparaasitology, Roman period, lead coffin
For the past century, paleoparasitology has been studying ancient parasites from humans and animals in archaeological and paleontological remains [
1]. In 1910, the paleopathologist Sir Marc Armand Ruffer was the first to publish observations of ancient parasites in 2 Egyptian mummies (
Schistosoma haematobium calcified eggs) [
2]. After this paper, paleoparasitology focused mainly on the analysis of human remains, but studied materials diversified rapidly and were soon sampled from all contexts potentially containing parasite residues, like latrines, cesspits, occupation layers, or sewers. For the Roman period, burials and human remains, such as mummies or skeletons, are poorly represented compared to hollowed structures (respectively 13.34% vs 78.4% calculated on 396 vestiges) [
3]. Analyses conducted on lead coffins are even scarcer. This type of burial is of particular interest due to its ability to preserve organic matters, unlike wooden coffins or shrouds. Moreover, outer elements cannot pollute the coffin (except in case of looting), and there is thus little doubt that the potentially recovered parasitic residues belong to the individuals inside the coffin.
To our knowledge, up until now, paleoparasitological investigations have only been performed on 2 burials of this type. In the UK, 1 individual from a Roman lead coffin with plaster (gypsum) was positive for
Ascaris lumbricoides and
Trichuris trichiura [
4]. In France, 1 lead coffin was studied prior to the present work. It was recovered from the site of Evreux (Late 3rd c. CE) but tested negative for parasites [
3]. Our paper presents the study of 2 new lead coffins discovered in France and aimed to provide additional paleoparasitological data for the Roman period.
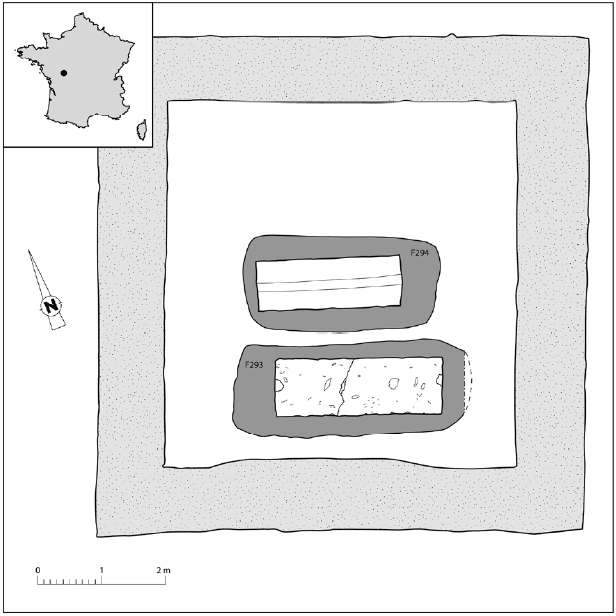
The archaeological site of Jaunay-Clan in France corresponded to an antique funerary area, principally composed of a funeral pyre dated to the 3rd c. CE and a mausoleum containing 2 rectangular calcareous sarcophagi dated to the 3rd–4th c. CE (
Fig. 1). In each sarcophagus, an individual lay in a rectangular lead coffin. The first, registered as F294, was situated in the mausoleum centre and corresponded to an immature individual (aged approximately between 9 and 14 years) of undetermined gender. The second, registered as F293, was situated in the south and corresponded to an aged adult male (
Fig. 2). The discovery of these 2 burials was exceptional due to the scarcity of mausoleums with sarcophagi in France. Moreover, organic matter was well preserved inside the coffins. For these reasons, pluridisciplinary research was conducted, including anthropology, paleogenetics, paleoparasitology, palynology, textile studies, chemical analyses, and a study of the sarcophagi (Collective Research Program on the burials of Jaunay-Clan directed by Maxence Segard). The aim was to provide information about funeral practices (treatment of the body, offerings, container study, etc) and about individuals (paleopathologies, possible family links, etc). Paleoparasitological analysis is in keeping with these topics and can provide information about health status, life conditions (hygiene, diet...), and funeral practices.
Our analyses here aimed to retrieve preserved gastrointestinal parasite eggs using light microscopy. Twelve samples taken from the interior of the 2 coffins were analyzed (
Table 1). For each individual, samples came from the pelvic area, bone surfaces, and from the bottom of the coffin. In addition, control samples were taken from below the feet of both individuals.
Sample preparations were all performed using the RHM (rehydration-homogenization-microsieving) standard protocol used in our laboratory [
5]. First, 5 g of each sample was rehydrated for 7 days in a solution composed of 50 ml of 0.5% trisodium phosphate (TSP) and 50 ml of 5% glycerinated water. To avoid fungi or algae development during the rehydration step several drops of 10% formalin solution were added. Then, the material was crushed in a mortar and placed in an ultrasonic device for 1 min (50/60 Hz). The suspension was strained through 315 μm, 160 μm, 50 μm, and 25 μm meshes under a constant flow of water. The last 2 screenings were placed in a PVC tube (4 ml) with formalin drops. Finally 20 slides (22×22 mm) with 15 μl (total 300 μl) of each sample were examined under the optical microscope. Parasite residues were identified and counted.
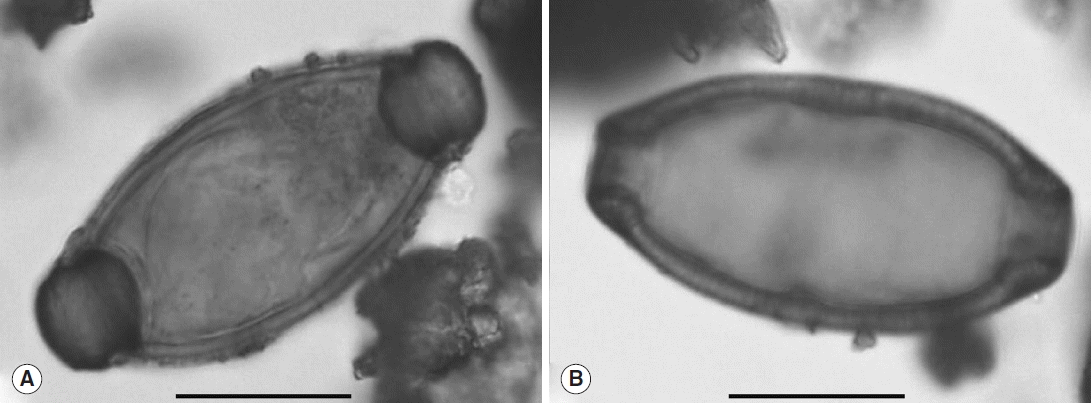
Only 1 individual yielded positive results. The analysis of sediment taken from the surface of the sacrum of the individual in coffin F293 (sample JC_FC_689,
Table 1) revealed the presence of 2 ovoid eggs, characterized by 2 polar plugs giving them a characteristic lemon shape (
Fig. 3). Egg sizes without polar plugs were 46.4 μm long and 25.2 μm wide for the first (
Fig. 3A) and 54.5 μm long and 26.4 μm wide for the second (
Fig. 3B). Because of the size (consistent with current data) and the archaeological context, these eggs were identified as the human whipworm,
T. trichiura. All samples from coffin F294 were negative.
The adult human whipworm measures between 30 and 50 mm long and is located in the intestines. Infection occurs through the accidental ingestion of fertile eggs polluted in soil, food, or drinking water. This disease is also linked to poor corporal hygiene, also called fecal peril (e.g. unwashed hands used to prepare food), the use of human fecal matters to fertilize crops, and to poor organic waste management (e.g. insufficiently cleaned roads and soils) [
6]. In humans, the presence of whipworm is generally asymptomatic but in case of massive infection, multiple symptoms may occur, such as dysentery, anemia, and growth retardation. Finger clubbing and rectal prolapse may also occur [
7].
Whipworm associated with human remains (skeletons or mummies) is not new for the Roman period. Eight individuals from 7 archaeological sites all located in the north-western part of the Roman Empire tested positive for the presence of
T. trichiura (
Table 2). Szidat in 1944 was the first to observe this parasite in Karwinden Man, a bog body from Poland dated to 500 CE [
8]. In 1986, Jones identified
T. trichiura eggs in the intestinal content of Lindow Man, a second bog body recovered in England (Cheshire) and dated between 2 BCE and 119 CE [
9]. One year later in 1987, Jones [
4] described human whipworm eggs in an individual buried during the Roman period at the site of Poundbury (Dorset), in a lead coffin with plaster packing [
4]. In France, in 1996, Rousset et al. [
10] identified the parasite at the site of Bobigny, in a sediment sample extracted from a burial dated between the 2nd c. BCE and the 1st c. CE. In 2013, Searcey et al. [
11] found
T. trichiura eggs in the digestive content of Zweeloo Woman, another bog body from the Netherlands, dated by radiocarbon between 78 cal AD and 233 cal AD [
11]. Finally in 2015 in France, during his PhD, Dufour [
3] observed human whipworm in a relegation burial from the site of Beauvais, dated between the mid-2nd c. and the 3rd c. CE, and mentioned the parasite in the abdominal cavity of 2 individuals dated to the 2nd c. CE buried at the site of Evreux.
These occurrences show the presence of the whipworm disease in humans during the whole Roman period. Practices such as the use of fecal matters for crop fertilization [
12] and general insalubrity in cities favored the development of geohelminthiases like trichuriasis or ascariasis in the Roman population. Public baths and the use of pigs to clean the streets were not sufficient to curb these parasitoses [
3,
13]. Early texts also mentioned the reality of parasitic diseases and sometimes described intestinal parasites, such as roundworms and tapeworms [
3]. Symptoms similar to those of dysentery, which can occur during massive parasite infections, were mentioned by the Roman encyclopedist Aulus Cornelius Celsus (c. 25 BCE-c. 50 CE) [
14].
Due to the low number of eggs retrieved from the individual in coffin F293, it was not possible to accurately determine the parasitic load, and it is possible that the number of adult parasites was low. However, biological processes occurring after death (thanatomorphose) and funerary practices could explain the presence of a single taxon and the scarcity of the observed eggs. Indeed, after a few hours and days, urine and feces expulsion takes place due to the loosening and laxity of the sphincters, followed by pressure of the putrefactive gases [
15,
16]. In this way, some of intestinal parasites are eliminated from the body. In addition, Roman funerary practices are also known through ancient texts. In particular, after the exposure of the dead (from a few hours for the poorest up to several days for the richest), the body was washed with warm water, scented and dressed before being buried [
17]. This preparation may contribute to eliminating some of the helminths and their markers. These same practices may also explain the negativity of the second individual. It is also possible that the individual was not parasitized.
Anthropological analysis showed no
cribra orbitalia on the skull of individual F294 [
18]. Although they are not specific signs,
cribra orbitalia and porotic hyperostosis on the skull vault can be the result of metabolic disorders (anemia) due to the presence of intestinal parasites [
7,
19]. The absence of these signs could point to the absence of parasites. Concerning the individual from coffin F293, from which the whipworm eggs were retrieved, the craniofacial bones were too damaged to detect potential osteological lesions [
20].
The present study provided the first positive results from a Roman lead coffin in France. This leads to a new mention of the reality of whipworm disease in humans during the Roman period. It completes the data for the end of this period.
The omnipresence of
Trichuris in archaeological material during the Roman period provides evidence of how lifestyles at that time were propitious to the development of such soil-transmitted parasites. With roundworm disease, or ascariasis, trichuriasis is the second most represented helminthiasis in the Roman world. Its presence reached 80% of all analyzed vestiges [
3]. It demonstrated that public sanitation measures were not effective enough to avoid such parasitic disease development [
13]. However, a recent review of the paleoparasitological data of the studied vestiges dated to the Roman period pointed out both geographical and chronological hiatuses. Indeed, the majority of the studied sites were localized in the northwestern part of the Roman Empire (87.5% of the 80 reviewed sites). Studies in other parts are scarcer, except in the Middle East region with analyses performed in Egypt, Sudan, Palestine, and Israel. A chronological hiatus is also to be noted, while the majority of the studied sites were dated between the 1st c. and the 3rd c. CE. Information from the beginning and the late Roman period is thus rather scant [
3]. Future research may complete these underexplored areas and periods for human remains as well as other vestiges. Finally, in addition to microscopic analyses, other parasitic markers could be tested to complete our knowledge of diseases in the Roman world, such as ancient DNA [
21,
22] and paleoantigens [
23,
24].
Notes
-
CONFLICT OF INTEREST
We declare that we have no conflict of interest related to this study.
ACKNOWLEDGMENTS
We wish to thank our colleagues from the Collective Research Program on the burials of Jaunay-Clan for discussions about funeral practices and archaeological contexts.
Fig. 1Location of the site of Jaunay-Clan in France and plan of the mausoleum (plan: Archeodunum modified by B. Dufour).

Fig. 2South sarcophagus F293 with the individual in the lead coffin (photo: Archeodunum).

Fig. 3Eggs of T. trichiura observed in sample JC_FC_689. Scale bars=20 μm (photos: B. Dufour).
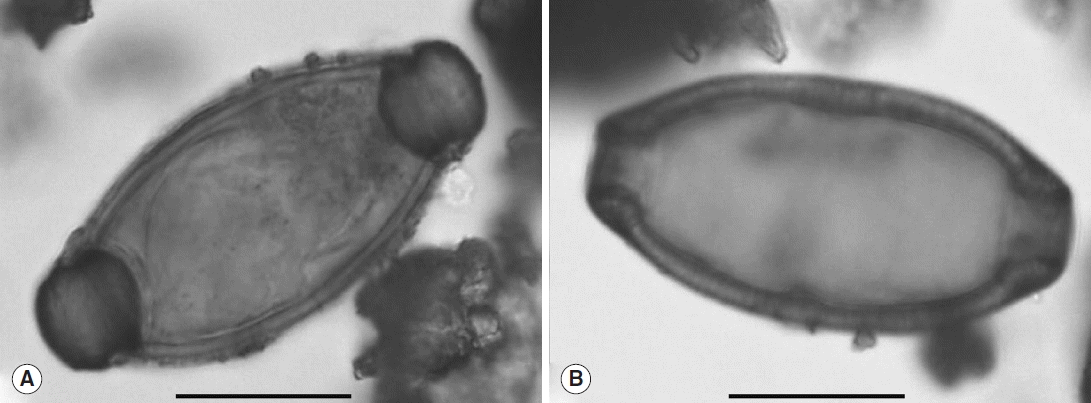
Table 1Description of the studied material and results
Table 1
|
Structure |
Samples |
Description |
Trichuris trichiura
|
|
South sarcophagus F293 |
JC_FC_536 |
Sediment from the bottom of the coffin below the feet |
- |
|
JC_FC_553 |
Organic matter from the surface of the right pubis/ischium |
- |
|
JC_FC_641 |
Sediment from the surface of the left coxal bone |
- |
|
JC_FC_689 |
Sediment from the surface of the sacrum |
2 |
|
JC_FC_690 |
Sediment from below the right coxal bone |
- |
|
JC_FC_691 |
Sediment from the surface of the last lumbar vertebrae |
- |
|
|
Central sarcophagus F294 |
JC_FC_181 |
Element from the surface of the sacrum (S2–S3) |
- |
|
JC_FC_182 |
Sediment from below the last lumbar vertebrae (L4–L5) |
- |
|
JC_FC_183 |
Sediment from the bottom of the coffin between coxal bones and sacrum, between the two pubis |
- |
|
JC_FC_185 |
Sediment from the surface of coxal bones, on the right and left iliums |
- |
|
JC_FC_194 |
Sediment from below the sacrum, along the median edge of the left ilium |
- |
|
JC_FC_304 |
Sediment from the bottom of the coffin below the feet |
- |
Table 2Summary of published data relating to Trichuris trichiura eggs in human remains during the Roman period (including the present study)
Table 2
|
Site names |
Countries |
Datations/periods |
Sample natures |
No. of individuals |
Origin structure |
Nature of remains |
Data origin |
|
Bobigny |
France |
2nd c. BCE - 1st c. CE |
S |
1 |
Burial |
Skeleton |
[10] |
|
Lindow Man |
UK |
2 BCE - 119 CE |
DC |
1 |
Bog body |
Mummy |
[9] |
|
Zweeloo Woman |
the Netherlands |
78 cal AD - 233 cal AD |
DC |
1 |
Bog body |
Mummy |
[11] |
|
Evreux |
France |
2nd c. CE |
S |
2 |
Burial |
Skeleton |
[3] |
|
Beauvais |
France |
Mid 2nd c. - 3rd c. CE |
S |
1 |
Relegation burial |
Skeleton |
[3] |
|
Jaunay-Clan |
France |
3rd c. - 4th c. CE |
S |
1 |
Burial in lead coffin |
Skeleton |
[3] |
|
Karwinden Man |
Poland |
500 CE |
DC |
1 |
Bog body |
Mummy |
[8] |
|
Poundbury |
UK |
Roman Period |
S |
1 |
Burial in lead coffin |
Skeleton |
[4] |
References
- 1. Dutour O. Paleoparasitology and paleopathology. Synergies for reconstructing the past of human infectious diseases and their pathocenosis. Int J Paleopathol 2013;3:145-149.
- 2. Ruffer MA. Note on the presence of “Bilharzia haematobia” in Egyptian mummies of the twentieth dynasty. Br Med J 1910;16:65.
- 3. Dufour B. Synthèse de données et nouvelle contribution à l’étude des parasites de l’époque romaine, et apports méthodologiques de l’extraction des marqueurs au traitement des résultats. Doctoral dissertation, Université de Franche-Comté. Besançon, France: University of Bourgogne Franche Comte; 2015. 361.
- 4. Jones AKG. Parasitological investigations on samples of organic material associated with human burials at the roman inhumation cemetery at Poundbury, Dorset (site code PC72-76). York, UK. Historic Buildings and Monuments Commission for England; 1987, No.: AML 40/87.
- 5. Dufour B, Le Bailly M. Testing new parasite egg extraction methods in paleoparasitology and an attempt at quantification. Int J Paleopathol 2013;3:199-203.
- 6. Euzéby J. Risques parasitaires liés aux déjections d’origine humaine et animale manipulées ou épandues le péril fécal et le problème de l’eau. Tampa, USA. Institut Romark pour la recherche médicale; 2002, p 307.
- 7. Roberts LS, Schmidt GD, Janovy J, Gerald D. Schmidt & Larry S Roberts’ Foundations of Parasitology. 8th ed. Boston, USA. McGraw-Hill Higher Education; 2009, p 701.
- 8. Szidat L. Uber die Erhaltungsfähigkeit von Helmintheneiern in Vor- und Frühgeschichtlichen Moorleichen. Z Parasitenkd 1944;13:265-274.
- 9. Jones AKG. Parasitological Investigations on Lindow Man. In Stead IM, Bourke JB, Brothwell D eds, Lindow Man, The Body in the Bog. London, UK. Book Club Associates; 1986, pp 136-139.
- 10. Rousset JJ, Heron C, Metrot P. Helminthoses humaines chez les Gaulois. His des Sci Med 1996;30:41-46.
- 11. Searcey N, Reinhard KJ, Egarter-Vigl E, Maixner F, Piombino-Mascali D, Zink AR, van der Sanden W, Gardner SL, Bianucci R. Parasitism of the Zweeloo Woman: Dicrocoeliasis evidenced in a Roman period bog mummy. Int J Paleopathol 2013;3:224-228.
- 12. Coulon G. Les villas gallo-romaines. Rennes, France. Ouest-France; 2005, p 31.
- 13. Mitchell PD. Human parasites in the Roman World: health consequences of conquering an empire. Parasitology 2016;8:1-11.
- 14. Penso G, Rampa MM. La Conquête du monde invisible: parasites et microbes à travers les siècles. Paris, France. R. Dacosta; 1981, p 379.
- 15. Karmakar RN. Forensic Medicine and Toxicology. Kolkata, India. Academic Publishers; 2007, p 476.
- 16. Charlier P. Ostéo-archéologie et techniques médico-légales: tendances et perspectives: Pour un ‘Manuel pratique de paléopathologie humaine’. Paris, France. De Boccard; 2008, pp 55-69.
- 17. Poux M. De la veillée au tombeau. In Goudineau C, Blaizot F, Fellague D, Golvin JC eds, Rites funéraires à Lvgdvnvm. Paris, France. Editions Errance; 2009, pp 25-46.
- 18. Dutour O, Naji S. Paléopathologie osseuse. Segard M. Jaunay-Clan – Vienne (86), Sous Clan 2 / ZAC des Grands Champs Rapport d’opération d’archéologie préventive. Chaponnay, France. Archéodunum; 2013, pp 187-188.
- 19. Walker PL, Bathurst RR, Richman R, Gjerdrum T, Andrushko VA. The causes of porotic hyperostosis and cribra orbitalia: a reappraisal of the iron-deficiency-anemia hypothesis. Am J Phys Anthropol 2009;139:109-125.
- 20. Chapelain de Sereville-Niel C, Ancel M-J, Charbouillot S, Rousseau C. Description et observations taphonomiques. Segard M. Jaunay-Clan-Vienne (86), Sous Clan 2 / ZAC des Grands Champs Rapport d’opération d’archéologie préventive. Chaponnay, France. Archéodunum; 2013, pp 169-175.
- 21. Oh CS, Seo M, Chai JY, Lee SJ, Kim MJ, Park JB, Shin DH. Amplification and sequencing of Trichuris trichiura ancient DNA extracted from archaeological sediments. J Archaeol Sci 2010;37:1269-1273.
- 22. Côté NML, Daligault J, Pruvost M, Bennett EA, Gorgé O, Guimaraes S, Capelli N, Le Bailly M, Geigl EM, Grange T. A new high-throughput approach to genotype ancient human gastrointestinal parasites. PLoS One 2016;11:e0146230.
- 23. Le Bailly M, Bouchet F. A first attempt to retrace the history of dysentery caused by Entamoeba histolytica. Sanitation, Latrines and Intestinal Parasites in Past Populations. Surrey, UK. Ashgate edition; 2015, pp 219-228.
- 24. Le Bailly M, Maicher C, Dufour B. Archaeological occurrences and historical review of the human amoeba, Entamoeba histolytica, over the past 6000 years. Infect Genet Evol 2016;42:34-40.