Prevalence and Molecular Characterization of Intestinal Trichomonads in Pet Dogs in East China
Article information
Abstract
The trichomonad species Tritrichomonas foetus and Pentatrichomonas hominis were recently detected in the feces of dogs with diarrhea. However, little information is available on the prevalence and pathogenicity of these parasites in the canine population. Therefore, the aim of this study was to determine the prevalence and molecular characterization of trichomonads infecting pet dogs in Anhui and Zhejiang provinces, east China. In total, 315 pet dogs, with or without diarrhea, from 7 pet hospitals were included in this epidemiological survey. Microscopy and PCR detected P. hominis in 19.7% (62/315) and 31.4% (99/315) of fecal samples, respectively. T. foetus infection was detected in 0% (0/315) of samples with microscopy and in 0.6% (2/315) with PCR. The prevalence of P. hominis was significantly higher in young dogs (≤12 months) than in adult dogs (>12 months), and was significantly higher in diarrheic dogs (50.6%) than in non-diarrheic dogs (24.3%; P<0.05). Infection with T. foetus did not correlate with any risk factors evaluated in this study. A sequence analysis of the P. hominis PCR products showed minor allelic variations between our sequences and those of P. hominis strains from other hosts in different parts of the world. Type CC1 was the most common strain in dogs in east China. The internal transcribed spacer 1 (ITS1)-5.8S rRNA gene sequences from the 2 T. foetus isolates detected in this study displayed 100% identity and were homologous to the sequences of other strains isolated from domestic cats in other countries.
INTRODUCTION
Trichomonads consist of both pathogenic and presumably non-pathogenic species and are frequently encountered in veterinary medicine [1,2]. These single-celled obligate organisms are characterized morphologically by multiple anterior flagella and a single recurrent flagellum that functions as an undulating membrane [3]. They commonly inhabit warm, moist, anaerobic locations within the gastrointestinal and genitourinary tracts of a variety of vertebrate and invertebrate hosts [2,4,5]. Two trichomonad species found in dogs have received scientific attention, Tritrichomonas foetus (family Tritrichomonadidae) and Pentatrichomonas hominis (family Trichomonadidae) [1,2,6–10].
T. foetus is mainly known as the causative agent of bovine trichomoniasis, which can lead to infertility and occasionally abortion [3,11]. A recent study suggested that T. foetus is the primary cause of chronic large-bowel diarrhea in domestic cats [12,13]. T. foetus has been identified as a synonym of T. suis, which is recognized as a facultative pathogen in the large intestine of pigs [14,15].
In contrast, P. hominis has frequently been reported in dogs [1,2,6–10] and is presumed to be a commensal organism that may overgrow opportunistically in dogs with diarrhea from other causes [1,2]. However, several authors have described P. hominis as the probable causative agent of gastrointestinal disturbances in children [16,17], and this protozoan has also been found occasionally outside its natural habitat, in patients with liver abscesses [18] or empyema thoracis [9,19]. This suggests the zoonotic potential of P. hominis and the existence of host-specific genotypes, as found among T. foetus isolates from cats and cattle [20,21].
The total number of dogs in China at present is vast, and the number of pet dogs alone approaches 200 million [22]. Pet dogs have played a vital role in human life in most cities and rural regions of China. Unfortunately, few data are available on the epidemiology of trichomonads in pets in China. Recently, our laboratory identified P. hominis infections in dogs [2] and investigated their prevalence in dogs in northeast China [10]. Given China’s vast territory, the occurrence of the parasite may vary greatly in different regions. Until now, no study has investigated T. foetus infections in dogs, and it is unknown whether P. hominis or T. fetus is more common in dogs in China. Therefore, the aims of the present study were (1) to determine the prevalence of trichomonads infecting pet dogs in east China; (2) to evaluate the risk factors for trichomonad infections in dogs; and (3) to investigate the genetic diversity of trichomonad isolates identified in the Chinese pet dog population.
MATERIALS AND METHODS
Sample collection and processing
A total of 315 fecal samples were collected between April and December 2013. The study animals were from 7 pet hospitals distributed in the cities Hefei (1), Xuancheng (1), Chuzhou (2), Bengbu (1), and Suzhou (1) of Anhui province, and in the city Hangzhou (1) of Zhejiang province (Fig. 1). The data used to evaluate the possible risk factors associated with infection were recorded with a written survey at the time of sample submission, and included the age, sex, source, fecal form classified as consistent (normal) or not consistent (pasty, poorly formed, or liquid), and medications used. Permission was obtained from the dog owners before the fecal samples were collected, and the experimental protocol was approved by the Animal Care and Welfare Committee of Anhui Science and Technology University. All fecal samples were submitted to the laboratory, stored at room temperature, and preliminarily screened within 3 hr with light microscopy. The remaining portions of all the samples were stored at 4°C until DNA extraction.
DNA extraction
Genomic DNA was extracted directly from a 200-mg sample of each preserved fecal specimen using the Stool DNA Kit (Tiangen, Beijing, China), according to the manufacturer’s instructions. The DNA samples were stored at −20°C until analysis.
PCR analysis of P. hominis and T. foetus
All the extracted fecal DNAs were subjected to single-tube nested PCR amplification of a 339-bp sequence of the partial 18S rRNA gene of P. hominis, using previously published reaction conditions and primer sequences [10]. Each fecal DNA was then subjected to another single-tube nested PCR targeting the ITS1-5.8S rRNA gene of T. foetus, as previously described [3,23]. To analyze the genetic diversity of T. foetus, the T. foetus-positive isolates identified in this study were amplified again using the trichomonad-specific sense primer TRICHO-F and antisense primer TRICHO-R targeting the ITS1-5.8S rRNA-ITS2 region, as previously described [19,24].
DNA sequence analysis
The secondary PCR products were analyzed by electrophoresis on a 1.5% agarose gel and visualized with ethidium bromide staining. The target PCR products were purified with a Biospin Gel Extraction Kit (Bioer, Hangzhou, China). The purified DNA fragments were directly sequenced in both directions on an ABI 377 automated DNA sequencer (Applied Biosystems, Foster City, California, USA) using the primer sets Th3/Th5 [10] or TRICHO-FBIS/TRICHO-RBIS [24]. The sequences were analyzed and aligned with trichomonad reference sequences available in databases, using the BioEdit v 7.1.3.0 software (Ibis Biosciences, Carlsbad, California, USA). Because many of the nucleotide sequences determined in this study were identical, only representative sequences have been deposited in GenBank under accession nos. KX136876-KX136894 for P. hominis and KX136895-KX136896 for T. foetus.
Statistical analysis
To explore the relationships between trichomonad infection and specific risk factors, statistical analyses were performed with a χ2 test for age and sex and with Fisher’s test for clinical symptoms, using SPSS for Windows (release 13.0 standard version, SPSS Inc., Chicago, Illinois, USA). Differences were considered statistically significant at P<0.05.
RESULTS
Prevalence of trichomonad infection
A total of 315 fecal samples from dogs in 7 pet hospitals were screened, and 62 (19.7%) and 99 (31.4%) were positive for P. hominis on microscopy and PCR, respectively (Table 1). The highest rates of P. hominis infection were 25.8% and 48.4%, on microscopy and PCR, respectively, in the Bengbu City. Among the samples that were P. hominis-positive on microscopy, 10 were not amplified with PCR, probably because either PCR inhibitors were present in the fecal samples, or the numbers of parasites were low in the fecal samples randomly selected for DNA extraction.
Two samples from 2 pet hospitals (located in the Bengbu City and the Hangzhou City) were positive for T. foetus on PCR, with an average infection rate of 0.6% (data not shown). Thus, the prevalence of T. foetus in the Bengbu City and the Hangzhou City was 3.2% and 1.00%, respectively. Two samples that were T. foetus-positive on PCR were negative on microscopy, and the 2 T. foetus-positive samples were both P. hominis-negative on microscopy and PCR.
Risk factors associated with infection and clinical signs
The mean age of the screened dogs was 24.0 months, whereas the mean ages of the dogs infected with P. hominis and T. foetus were 20.0 and 14.0 months, respectively. From our statistical analysis, P. hominis infection in pet dogs was significantly associated with 2 factors: clinical symptoms (diarrhea or no diarrhea) and age (≤12 or >12 months). However, the P. hominis infection rates in males and females did not differ significantly. Infection with T. foetus did not correlate with any risk factors evaluated in this study (Table 2).
Molecular characterization of trichomonad isolates
Nested PCR resulted in specific bands of approximately 339 bp (P. hominis) and 363 bp (T. foetus). All the trichomonad-positive samples were successfully sequenced. With the exception of isolate KX136880 (isolated from the Bengbu City), an alignment of the amplification products of the 18S rRNA gene demonstrated that the dog P. hominis isolates identified in this study and obtained from other hosts, including humans, cats, cattle, goats, and dogs had almost identical sequences (98.8–99.7%), and only differed from the reference sequence (AY 758392) at fewer than 5 variable positions. However, KX136880 showed only 95.6% identity and differed at 15 positions from no. AY758392 (Fig. 2). Interestingly, nucleotide 317 in all the 18S rRNA sequences from dogs in China was G, whereas it was C in the sequences from other hosts in other countries (Fig. 2). Among the 99 P. hominis-positive isolates identified in this study, the sequences of 62 (62.6%) from the 7 pet hospitals displayed 100% identity to type CC1 (KJ4 08929). The remaining 18 sequences isolated in this work were all different from the type CC1.

Alignment of the 18S rRNA sequences of the P. hominis isolates analyzed in this study and P. hominis strains of interest. Only the nucleotides that differ from those in the reference sequence (no. AY758392) are indicated. Dots represent the consensus sequence of all the P. hominis strains used in this figure.
For T. foetus, the 2 isolates of T. foetus identified in this study showed 100% homology. In the common part of our alignment, these sequences also showed 100% identity to the corresponding sequences in T. foetus strains isolated from domestic cats in the USA (AF466749, AF466750, and EU569309), Switzerland (JN006994), Norway (HM856630 and EF165538), Australia (GU170216 and HM046255), Brazil (KU680816), and France (JX960422). The sequences from the domestic cats and dogs differed only by a single-nucleotide polymorphism (SNP) from those isolated from cattle (JN106456 in China, and GU170220 in Australia) and pigs (KP012680 in Australia) in the ITS2 region (Fig. 3).
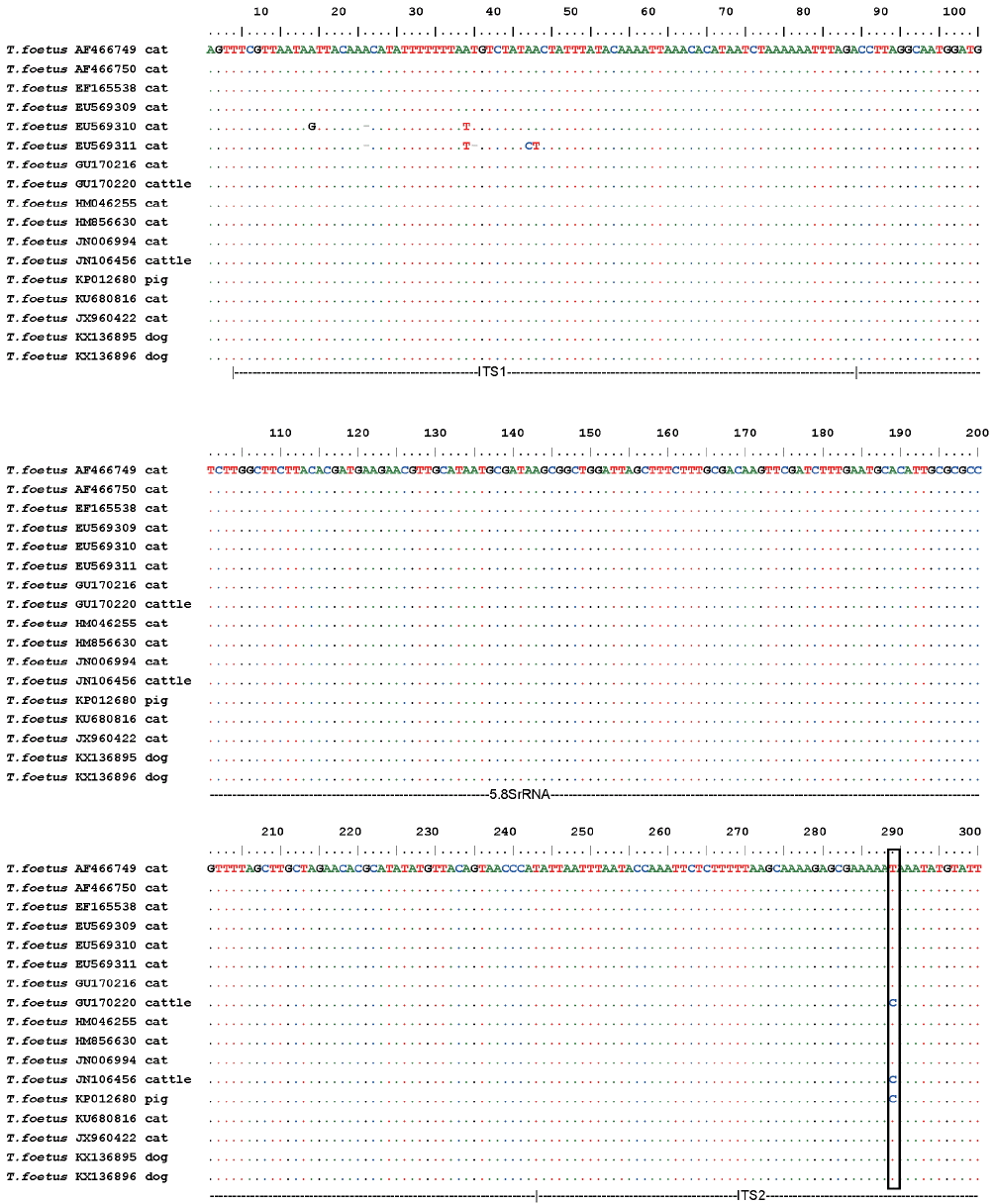
Alignment of the ITS1-5.8S rRNA-ITS2 sequences of the T. foetus isolates analyzed in this study and T. foetus strains of interest. Only the nucleotides that differ from those in the consensus sequence used are indicated. Dots represent the consensus sequence of all the T. foetus strains used in this figure, and dashes are gaps inserted to optimize the alignment. The black-boxed residues indicate differences in the ITS2 region.
DISCUSSION
Because it is difficult to distinguish different trichomonad species that share similar morphological features using light microscopy [25,26], molecular tools have been widely used for the identification of trichomonad species and strains. The 18S rRNA [26], ITS1-5.8S rRNA-ITS2 [27,28], and some protein-coding genes, such as glyceraldehyde 3-phosphate dehydrogenase (GAPDH), malate dehydrogenase (MDH), and enolase [29], are commonly described genetic markers. To our knowledge, the present study is the first to report the occurrence of T. foetus in pet dogs and confirms that P. hominis infection is more frequent than T. foetus infection in pet dogs in China. This finding provides objective data to support the previous assumption that trichomoniasis in dogs is most commonly attributable to P. hominis infection [1,7,9].
In this study, 99 samples were positive for P. hominis and 2 were positive for T. foetus when PCR was used, whereas only 62 were positive for P. hominis and none for T. foetus when microscopy was used because the trophozoites were rapidly destroyed in the feces or impurities in the feces interfered with the PCR reaction. These results clearly show that PCR is more sensitive than microscopy in detecting these parasites.
A study of the prevalence of intestinal trichomonads in breeding kennels in France found that 15.8% (34/215) of the puppies and 20.0% (5/25) of the breeding kennels tested were positive for trichomonads and that P. hominis was the only trichomonad infecting the canine population [9]. Our laboratory previously found that the prevalence of P. hominis infections in dogs in northeast China was 27.4% (69/252) [10]. In contrast, in this study, we detected a higher rate of P. hominis infections (31.4%) in pet dogs in east China than in northeast China.
In the present survey, the prevalence of P. hominis was significantly higher in dogs younger than 12 months (53.3%) than in dogs older than 12 months (27.8%; P<0.05). This result is consistent with previous studies by [6,7,9,10], which suggested that puppies were more susceptible to P. hominis infection than adult dogs, probably because their immune systems are immature [9]. Previous studies have reported that dogs presenting with P. hominis infections are generally younger than 9 months [6,7]. However, it is noteworthy that the mean age of the dogs infected with P. hominis was 20.0 months in the present study. The 2 dogs identified with T. foetus infections were also older than 12 months (mean age, 14.0 months). A similar observation was made previously [1], which reported that T. foetus-infected dogs ranged in age from 10 weeks to 10 years.
Only a handful of case studies or surveys, which have included only a limited number of animals, have reported that P. hominis is more frequent in diarrheic dogs than in non-diarrheic dogs [1,2,6,7]. In our study, P. hominis infection was significantly associated with abnormal feces, supporting the assertion that the liquid or semiliquid anaerobic environment created by diarrhea may favor the opportunistic overgrowth of P. hominis. This result is consistent with previous reports of T. foetus infections in cats [30]. Before the univariate risk factor analysis performed in this study, it was unclear whether the observed diarrhea was directly attributable to P. hominis infection or to the presence of other common enteropathogens can also cause clinical diarrhea. The pathogenic potential of P. hominis warrants investigation, as does the enteritic coinfection status of dogs infected with P. hominis [1,9].
In the present survey, T. foetus infection was identified infrequently. This result is quite similar to those found by Tolbert et al. [1] and Grellet et al. [9]. In contrast to P. hominis infection, T. foetus infection in dogs did not correlate with any of the risk factors evaluated in this study. This may be because the number of cases identified was low or because the number of animals included in the study was limited [31].
Of the 99 18S rRNA gene sequences determined for P. hominis isolates from dogs, 62.6% belonged to type CC1, as described previously by our laboratory [10], and no type CC2 (KJ408931) or type CC3 (KJ408928) was detected in the present study. These data suggest that type CC1 is most common in dogs in east China, which is quite similar to the distribution found in northeast China [10]. The remaining 18 sequences identified in this work differed from types CC1, CC2, and CC3, and may be new types of P. hominis. The few differences detected between the PCR products indicate that they were all derived from the same strain, and can be attributed to the expected variation within the multiple copies of the 18S rRNA genes in any given genome [9]. The high degree of similarity between the sequences isolated in this study and those of P. hominis strains from different hosts implies that they all belong to the same species [9]. Therefore, our data support the conclusion that the same P. hominis species colonizes the digestive tracts of several mammal hosts [17] and imply the potential zoonotic transmission of P. hominis between its human and animal hosts.
The ITS1-5.8S rRNA gene sequences of T. foetus obtained from 315 samples showed 100% identity, strongly suggesting that they were all derived from the same T. foetus strain. A comparative analysis with homologous sequences of T. foetus isolated from domestic cats in different countries also showed 100% identity. As previously described by [31], the sequences of the dog isolates determined in our study and all the sequences from domestic cat isolates currently available in GenBank show the same SNP in the ITS2 region relative to the T. foetus sequences from cattle (T for felids and C for bovids), which could distinguish the “cat genotype” from the “cattle genotype” [20,21]. This result suggests that domestic cats represent an important source of infection for dogs, given the high frequency of contact between dogs and cats in China.
In conclusion, we have shown the occurrence of T. foetus in pet dogs for the first time and confirmed that P. hominis is a widespread parasite in pet dogs in east China. A comparative analysis of the 18S rRNA sequences from numerous isolates suggested that P. hominis has high zoonotic potential. A sequence alignment of the ITS1-5.8S rRNA of T. foetus revealed the complete genetic identity between feline isolates and the canine isolates in our study, and confirmed that domestic cats may represent an important source of infection for dogs. In this study, the age and clinical symptoms of pet dogs were risk factors for P. hominis infection. However, because other enteropathogens that can cause diarrhea are frequently also present, further experimental studies are required to confirm the pathogenicity of trichomonads in dogs.
ACKNOWLEDGMENTS
We thank all staff for their assistance with this study. This study was supported by the National Natural Science Foundation of China (no. 30970322), the Seventh Installment of Innovation Team for “115” Industry of Anhui Province, the Key Program of Anhui Province Education Department (nos. KJ2014ZD09 and KJ2015A189), and the Key Projects of Anhui Province University Outstanding Youth Talent Support Program (no. gxyqZD2016220), the Key Discipline Construction Program of University of Science and Technology of Anhui (no. AKZDXK2015A04) and the Key Program of Anhui Science and Technology University (no. ZRC2016478). We declare that the experiments complied with the laws of China when they were performed.
Notes
CONFLICT OF INTEREST
The authors declare that they have no competing interests.