Apodemus agrarius as a new definitive host for Neodiplostomum seoulense
Article information
Abstract
A total of 1,496 rodents and insectivores were live-trapped at Yeoncheon-gun (n = 351), Paju-shi (804), and Pocheon-gun (343), Gyeonggi-do (Province), and examined for intestinal helminths, including Neodiplostomum seoulense, seasonally from December 2004 to September 2005. Six species of rodents, including Apodemus agrarius (1,366), Mus musculus (32), Micronytus fortis (28), Eothenomys regulus (9), Micronys minutus (6), and Cricetulus triton (3), and 1 species of insectivores Crocidura lasiura (54) were collected. A total of 321 adult N. seoulense were collected from 19 (1.4%) A. agrarius. The worm burden ranged from 1 to 101 per A. agrarius (mean; 16.9). No N. seoulense was observed in other rodent or insectivore species examined. The infection rate during autumn (4.5%) was higher than those during spring (0.8%), summer (0.8%), and winter (0.5%). The average number of N. seoulense in infected A. agrarius was the highest in spring (66.0 specimens), followed by autumn (15.2), winter (4.5), and summer (3.3). This study first confirms that A. agrarius is a natural definitive host for N. seoulense, and demonstrates that the infection rates and intensities vary seasonally and geographically.
Neodiplostomum seoulense (Digenea: Neodiplostomidae) was described first from the small intestine of house rats (Seo et al., 1964a) and later in a total of 26 documented human infections (Seo et al., 1982; Hong et al., 1984, 1986). Several species of frogs and tadpoles were found to be the second intermediate hosts, and snakes, Rhabdophis tigrina, take the role of a paratenic host and the source of human infections (Hong, 1982; Seo et al., 1988). A species of freshwater snail, Hippeutis cantori, is known to be the first intermediate host (Seo et al., 1988; Chai and Lee, 2002).
With regard to the natural definitive host for N. seoulense, limited information is available. Several surveys on intestinal parasites of rodents in the Republic of Korea (South Korea) suggested that N. seoulense was a species having one natural definitive host, R. norvegicus (Seo et al., 1964a, b, 1981; Yong et al., 1991), and their distributions were limited to certain parts of South Korea, i.e., Seoul, Namyangju, Yongin, Youngwol and Jungwon (Seo et al., 1964a, b, 1981). No N. seoulense was found in other rodent species, including Apodemus agrarius, Micronys minutus, Micronytus fortis and Mus musculus, or an insectivore, Crocidura russula, which were collected from Koyang-gun, Gyeonggi-do, and Iri-si and Iksan-gun, Jeollabuk-do (Yong et al., 1991). However, in mountainous and non-urban areas (agricultural land and military training sites), commensal R. norvegicus is not commonly collected. This strongly suggests that field rodents, including A. agrarius, and other small mammals may be involved in the maintenance of N. seoulense life cycle in sylvatic environments.
Along the northern boundary of Gyeonggi-do near the demilitarized zone (DMZ), where civilians are prohibited entry, military training sites are located. The natural plant and animal ecology along the training sites perimeter and hills, where military activities are limited, is well preserved. The present study was undertaken to determine the infection status of small mammals, including rodents and insectivores in areas near the DMZ, with N. seoulense, as a part of a comprehensive rodent surveillance program in 3 localities of northern Gyeonggi-do.
A total of 1,496 small mammals were caught using Sherman traps (3 × 3.5 × 9" folding traps; H.B. Sherman, Tallahassee, Florida, USA) as described by Chai et al. (2007) from 3 areas in Gyeonggi-do, i.e., Yeoncheon-gun (n = 351), Paju-shi (804) and Pocheon-gun (343), at military field training sites near the DMZ. Animals were trapped seasonally from December 2004 through September 2005. Total 7 species, including 6 species of rodents, i.e., A. agrarius (1,366), Cricetulus triton (3), M. musculus (32), M. fortis (28), Eothenomys regulus (9) and M. minutus (6), and 1 species of insectivores, i.e., Crocidura lasiura (54), were collected. Animals were euthanized in accordance with an approved animal use protocol under biosafety level 3 (BSL-3) laboratory conditions.
Gastrointestinal organs, including the stomach, the small intestine, and the large intestine (from the stomach to the end of the rectum) were removed and preserved in 70% alcohol until examination. Gastrointestinal tracts were opened and examined for intestinal helminths under a stereomicroscope (Chai et al., 2007). Trematodes collected were placed on a microscope slide with a coverslip, stained with Semichon's acetocarmine, and identified using a research microscope. Data on nematodes, cestodes, and trematodes other than Plagiorchis muris (Chai et al., 2007) and N. seoulense (this paper) will be published separately.
A total of 321 adult N. seoulense were collected from 19 (1.4%) of 1,366 A. agrarius (Table 1). Worm burdens in each infected A. agrarius ranged 1 to 101 (mean; 16.9). N. seoulense was not observed in other rodent and insectivore species captured. Seasonal variation in infection rates and intensity was observed. The infection rate was higher in A. agrarius captured in autumn (4.5%) than those in spring (0.8%), summer (0.8%) and winter (0.5%) trapping periods (Fig. 1). The mean number of worms (= worm burden) in N. seoulense-infected A. agrarius was the highest in spring (66.0), followed by autumn (15.2), winter (4.5), and summer (3.3) (Fig. 1). In spring, infected A. agrarius was found only in Yeoncheon-gun, in winter, only in Paju-shi, and in summer, in Yeoncheon-gun and Pocheon-gun (Fig. 2). In autumn, infected A. agrarius was found in all surveyed areas (Fig. 2).

Infection rates of Neodiplostomum seoulense among small mammals captured from northern Gyeonggi-do (Province), near the demilitarized zone (DMZ)
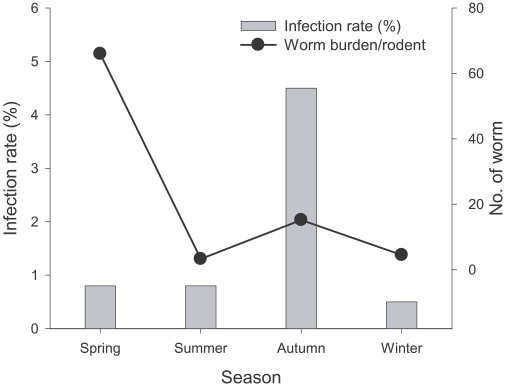
Seasonal changes in infection rates and mean worm burdens in A. agrarius. Spring = March (n = 255); Summer = June (495); Fall = September (247); and Winter = December (369).

Seasonal and geographical distribution of Apodemus agrarius infected with Neodiplostomum seoulense. Surveyed areas include Paju-shi, Yeoncheon-gun, and Pocheon-gun, Gyeonggi-do (Province). A: spring, B: summer, C: autumn, and D: winter. DMZ: demilitarized zone (heavy black line). Closed circle means 1 infected A. agrarius.
In the present study, A. agrarius was identified as a new definitive host for N. seoulense in northern districts of Gyeonggi-do. Infection rates in A. agrarius were relatively low, ranging from 0.5% to 4.5% seasonally. The overall mean worm burden was also relatively low, i.e., 16.9 per mouse, with heavy infections of over 50 specimens (101, 70, and 61) only in 3 mice.
Adult N. seoulense reside in the duodenum, and if the worm burden is high, the location of worms extends to the jejunum and ileum (Hong, 1982). They evoke mechanical damages in the mucosa, and cause villous atrophy, with blunting and fusion of villi, and crypt hyperplasia in experimental mice (Lee et al., 1985). In the first documented human case, the patient suffered from acute epigastric discomfort and pain with diarrhea and fever, and admitted to the Seoul National University Hospital through the emergency room (Seo et al., 1982).
It has been shown that mice heavily infected with N. seoulense can be fatal within a month (Huh et al., 1988; Kook et al., 1998, Chai et al., 2000). When several strains of laboratory mice, including mast cell-deficient W/Wv, their normal littermates +/+, C57BL/6, C3H/HeJ, and a hybrid (BALB/cA × C3H/HeJ) F1 mice, were infected with 200 metacercariae, all mice died by day 23 post-infection (Kook et al, 1998). Even a small infection dose of 25 metacercariae was highly lethal to C3H/HeJ mice (Kook et al., 1998). Considering this high pathogenicity of N. seoulense in laboratory mice, it is suggested that high worm burdens in the field mouse A. agrarius may also be harmful and lethal to the host and only mice with low to moderate infections may survive in the field. However, lethality of mice may be enhanced by other zoonotic infections, e.g., hantavirus infection, scrub typhus, and leptospirosis, that are relatively common in A. agrarius in the surveyed areas (JW Song, personal communication).
A nationwide survey of frogs and tadpoles in South Korea demonstrated their high infection rates and intensities with N. seoulense metacercariae (Hong et al., 1985). Therefore, tadpoles and frogs are suggested to be the main source of N. seoulense infection in A. agrarius (Lee et al., 1986; Seo et al., 1988). Snakes, in particular, the grass snake R. tigrina, were also reported to be infected with N. seoulense metacercariae (Hong et al., 1985). However, there is little possibility that snakes serve as the source of N. seoulense infection in A. agrarius, since snakes are a predator, rather than a prey, of A. agrarius.
The infection rates and worm burdens of N. seoulense in A. agrarius varied seasonally. The mean worm burden was the highest in spring, whereas the infection rate was the highest during the autumn sampling period. The ecology of the second intermediate hosts, i.e., tadpoles and frogs, may give an impact on these observed differences. During the summer months, many tadpoles emerge and develop into adult frogs. With the increased number of tadpoles and frogs during the summer months, rodents that prey on them may have an increased potential to become infected with N. seoulense, resulting in higher infection rates during the autumn season. If tadpoles or frogs continue to be in touch with water contaminated with N. seoulense cercariae for extended periods of time, they have greater opportunities to be infected with N. seoulense. Prior to hibernation, frogs will be frequently exposed to water and cercariae ingested would accumulate in their muscle tissues becoming metacercariae (Hong et al., 1985). Frogs emerging from hibernation that harbor metacercariae are subject to be preyed upon by wild animals, including rodents. The worm burden would be relatively high for wild animals that consume frogs heavily infected with N. seoulense metacercariae in spring, even though their infection rates were relatively low.
The geographical distribution of N. seoulense-infected A. agrarius increased over time from spring to autumn and was limited to Paju-shi during the winter season. As there is no evidence of A. agrarius migrating due to changes in environmental temperatures or other factors, this is most likely due to very low infections associated with high mortality rates of infected mice. N. seoulense was not found in the other 5 species of small mammals examined. Also in previous studies, N. seoulense was not collected from these species (Seo et al., 1964a; Yong et al., 1991). Hence, it may be possible that small mammal hosts, i.e., M. musculus, M. minutus, C. triton, M. fortis, E. regulus, and C. lasiura, are less suitable for N. seoulense infection than A. agrarius or R. norvegicus.
References
Notes
Funding for portions of this work was provided by the U.S. Department of Defense, Global Emerging Infections Surveillance and Response System, Silver Spring, Maryland, and the Armed Forces Medical Intelligence Center, Ft. Detrick, Maryland, USA.