Zoonotic Trematode Metacercariae in Fish from Yangon, Myanmar and Their Adults Recovered from Experimental Animals
Article information
Abstract
A survey was performed to investigate the infection status of zoonotic trematode (ZT) metacercariae in fish from a local market in Yangon City, Myanmar. A total of 264 fish (12 species) were collected through 4 times from December 2013 to June 2015. All collected fish were transferred to our laboratory on ice and examined by the artificial digestion method. More than 7 species of ZT metacercariae, i.e., Haplorchis taichui, H. pumilio, H. yokogawai, Centrocestus spp., Stellantchasmus falcatus, Pygidiopsis cambodiensis, and Procerovum sp. were detected. Metacercariae of H. taichui were collected in 58 (42.3%) out of 137 fish (5 species), and their average density was 42.9 per fish infected. Metacercariae of H. pumilio were detected in 96 (49.0%) out of 196 fish (9 species), and their average density was 23.6 per fish infected. H. yokogawai metacercariae were found in 40 (50.0%) out of 80 fish (5 species), and Centrocestus spp. metacercariae in 91 (50.8%) out of 179 fish (8 species), and their densities were 306 and 25.8 per fish infected, respectively. Metacercariae of S. falcatus and P. cambodiensis were detected only in mullets, Chelon macrolepis. A total of 280 Procerovum sp. metacercariae were found in 6 out of 12 climbing perch, Anabas testudineus. Morphological characteristics of adult flukes recovered from experimental animals were described. It has been first confirmed that fish from Yangon, Myanmar are commonly infected with various species of ZT metacercariae.
INTRODUCTION
Zoonotic trematode (ZT) infections are an important public health problem in many Asian countries, including Lao People’s Democratic Republic (Lao PDR), Vietnam, Cambodia, Thailand, the Philippines, China, Taiwan, and the Republic of Korea (Korea). Especially, fishborne trematodes (FBT) provoke a remarkable morbidity in residents of these countries and cause a serious economic damage in the industry of fish aquaculture [1–3]. Human FBT infections are mainly localized in riverside areas, where the riparian populations are infected by habitual consumption of raw and/or fermented fish containing infective larvae, i.e., metacercariae. It has been known that riverside areas in Southeast Asia, especially the Mekong river basin in Vietnam, Lao PDR, Cambodia, and Thailand, are highly endemic with FBT infections [4–8].
The Republic of the Union of Myanmar (Myanmar) is a sovereign state in the region of Southeast Asia, and bordered by India and Bangladesh to its west, Thailand and Lao PDR to its east, and China to its north and northeast. Administratively, it is divided into 7 states and 7 regions (formerly called divisions). Yangon City is the capital of the Yangon Region of Myanmar. It is the largest city and the most important socioeconomic center in Myanmar. Geographically, this city is located in the lower and coastal region of Myanmar, and it has diverse ethnic people [9]. We have been performing a health promotion project for the elementary schoolchildren of the vulnerable areas around Yangon area [10].
A survey on metacercarial infections in the second intermediate host, in combination with a survey on adult worm infections in humans, can be a useful index in the trematode epidemiology in a particular area. However, fecal examinations are not suitable to know the exact infection status in humans, since the egg identification in feces is very difficult in cases of mixed infections with small egg-sized trematodes, especially the liver flukes, as well as heterophyid, gymnophallid, and lecithodendriid flukes [5–7,11,12]. Therefore, investigation of metacercarial infections in the second intermediate host can provide a more valuable information on the trematode epidemiology. On the other hand, there are not so many studies on helminthic infections among the residents in Myanmar. Most of the previous studies were surveys of soil-transmitted helminthiases [13–15] except for one, Aung et al. [16], which reported the presence of the liver fluke (Opisthorchis viverrini) in Myanmar. Therefore, in the present study, we surveyed on the infection status of ZT metacercariae in fish purchased from a local market in Yangon, Myanmar. In addition, the morphological characteristics of adult flukes recovered from animals experimentally infected with these ZT metacercariae were described.
MATERIALS AND METHODS
We purchased a total of 264 fish (12 species) in a local fish market of Yangon, Myanmar, through 4 times (December 2013, June and December 2014, and June 2015). All collected fish were transferred on ice to the laboratory of Department of Parasitology and Tropical Medicine, Gyeongsang National University College of Medicine, Jinju, Korea. The length and weight of fish were individually measured and the species of fish identified with the aid of the FishBase website (http://www.fishbase.org/search.php) [17] (Table 1). Individual fish was finely ground with a mortar with pestle. The ground fish meat was mixed with artificial gastric juice, and the mixture was incubated at 36°C for 2 hr. The digested material was filtered with 1×1 mm of mesh, and washed with 0.85% saline until the supernatant became clear. The sediment was carefully examined using a stereomicroscope and then metacercariae were separately collected by their general morphological features. These collected metacercariae were categorized according to the size and morphological characteristics, and then the infection rate (%) and the intensity of infection (no. of metacercariae per fish infected) were calculated for each species of fish.
The identified metacercariae were experimentally infected to cats and hamsters to obtain adult flukes. At days 7–10 after infection, cats and hamsters were killed under anesthesia, and their small intestines were isolated and longitudinally opened with a pair of scissors in a beaker with 0.85% saline. The experimental animals were treated according to the guidelines of Institutional Animal Care and Use Committee (IACUC) in Gyeongsang National University, Jinju, Korea. Adult flukes were recovered in the sediment of intestinal contents which were diluted with 0.85% saline. Recovered worms were fixed with 10% formalin under a cover glass pressure, stained with Semichon’s acetocarmine, and observed under a light microscope equipped with a micrometer (OSM-4, Olympus Co., Tokyo, Japan).
RESULTS
Infection status of ZT metacercariae in fish from Yangon City
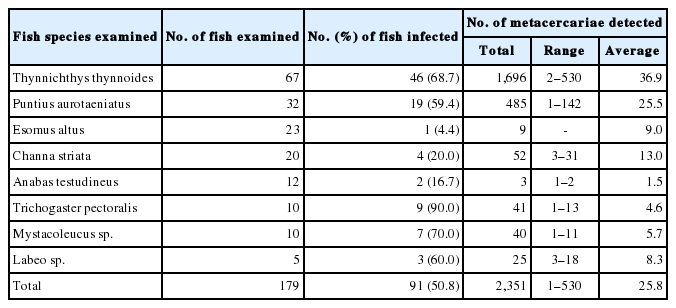
More than 7 species of ZT metacercariae, i.e., Haplorchis taichui, H. pumilio, H. yokogawai, Centrocestus spp., Stellantchasmus falcatus, Pygidiopsis cambodiensis, and Procerovum sp., were detected. The metacercariae of H. taichui were collected in 58 (42.3%) out of 137 fish (5 species), i.e., Thynnichthys thynnoides, Puntius aurotaeniatus, Esomus altus, Mystacoleucus sp., and Labeo sp., with their average density of 42.9 per fish infected. The infection status of H. taichui metacercariae by the fish species is revealed in Table 2. The metacercariae of H. pumilio were detected in 96 (49.0%) out of 196 fish (9 species), with their average density of 23.6 per fish infected. Their infection status by the fish species is designated in Table 3. H. yokogawai metacercariae were found in 40 (50.0%) out of 80 fish (5 species), and their density was 306 per fish infected. Centrocestus spp. metacercariae were found in 91 (50.8%) out of 179 fish (8 species), and their density was 25.8 per fish infected. The infection status of H. yokogawai and Centrocestus spp. metacercariae by the fish species is shown in Tables 4 and 5. The metacercariae of S. falcatus were detected in 15 (34.1%) out of 44 mullets, Chelon macrolepis, examined in December 2013 and 2014, and their density was 2.4 per fish infected. Total 38 metacercariae of Pygidiopsis cambodiensis were detected from only 1 mullet examined in December 2013. Total 280 (2–245: 46.7 in average) metacercariae of Procerovum sp. were detected in 6 out 12 climbing perch, Anabas testudineus, examined in June 2015.
Morphology of metacercariae detected (all measurement unit; μm)
Haplorchis taichui metacercariae (n=10) were elliptical, 192–218 (203 in average)×162–188 (172 in average) in size, had a baseball glove-shaped ventrogenital sac with 11–18 rodlets and an O-shaped excretory bladder occupying large portion of posterior body (Fig. 1A).
The metacercariae of zoonotic trematodes detected in fish from a local market of Yangon, Myanmar. (A) H. taichui: elliptical, 203×172 in average size (μm), had a baseball glove-shaped ventrogenital sac with 11–18 rodlets and an O-shaped excretory bladder. (B) H. pumilio: elliptical, 176×160 in average size, had 35–43 deer horn-like minute spines arranged in 1–2 rows around the ventrogenital complex, and an O-shaped excretory bladder. (C) H. yokogawai: round or elliptical, 196×188 in average size, had a U-shaped ventrogenital sac with 70–74 min spines, and an O-shaped excretory bladder. (D). Procerovum sp.: elliptical, 185×155 in average size, had yellow brownish pigment granules scattering in body area of intestinal bifurcation, a thin-walled bulb-like expulsor, and a D-shaped excretory bladder with grouped granules. (E1, E2) Centrocestus spp.: elliptical, 171×142 in average size, had 32 circumoral spines around the oral sucker arranged in 2 rows, and a X-shaped excretory bladder. (F) S. falcatus: elliptical, 225×165 in average size, had brownish pigment granules scattered in the body, and an O-shaped excretory bladder. (G) P. cambodiensis: elliptical, 242×194 in average size, and had an oral sucker, a pair of eyespots, ventral sucker, ventrogenital sac, and X-shaped excretory bladder (G). All scale bar is 50 μm.
Haplorchis pumilio metacercariae (n=10) were elliptical, 162–193 (176)×143–173 (160) in size, had 35–43 deer horn-like minute spines arranged in 1–2 rows around the ventrogenital complex, and an O-shaped excretory bladder occupying large portion of posterior body (Fig. 1B).
Haplorchis yokogawai metacercariae (n=10) were round or elliptical, 175–225 (196)×163–218 (188) in size, had an U-shaped ventrogenital sac with 70–74 min spines, and an O-shaped excretory bladder occupying large portion of posterior body (Fig. 1C).
Procerovum sp. metacercariae (n=10) were elliptical, 168–205 (185)×138–165 (155) in size, had yellow brownish pigment granules scattering in body area of intestinal bifurcation, a ventral sucker deflectively located from median, a thin-walled bulb-like expulsor, and a D-shaped (half-moon shaped) excretory bladder with grouped granules (Fig. 1D).
Centrocestus formosanus metacercariae (n=10) were elliptical, 152–195 (171)×122–157 (142) in size, had 32 circumoral spines around the oral sucker arranged in 2 rows, and a X-shaped excretory bladder occupying greater portion of posterior body (Fig. 1E1, 1E2).
Stellantchasmus falcatus metacercariae (n=10) were elliptical, 198–242 (225) by 135–192 (165) in size. Brownish pigment granules scattered in the worm body. Excretory bladder was O-shaped or not obviously seen in the posterior body (Fig. 1F).
Pygidiopsis cambodiensis metacercariae (n=10) were elliptical, 222–260 (242)×177–212 (194) in size, and had an oral sucker, a pair of eyespots, ventral sucker, ventrogenital sac, and X-shaped excretory bladder (Fig. 1G).
Morphology of heterophyid adults recovered from experimental animals
H. taichui (n=20, Fig. 2A): Body small, pear-shaped, 490–660 (591 in average) long, and 240–370 (298) wide, with greatest width at middle, the ovarian level. Oral sucker subterminal, 40–63 (48) by 53–75 (58). Pharynx subglobular or elliptical, 25–45 (34) by 18–38 (24). Esophagus short, 80–138 (106) in length. Ventrogenital sac small with 12–18 rodlets, baseball glove-shaped, 50–80 (64) by 40–63 (53). Seminal vesicle saccular, bipartite. Ovary spherical or subspherical, 45–95 (64) by 43–88 (63), dextral to midline. Seminal receptacle ellipsoidal, lying the right-side of ovary. One testis globular or subglobular, 113–188 (146) by 120–183 (148), lying posterior 1/4 of body. Uterus with eggs occupying from anterior 1/3 to posterior end, most of hind-body. Vitellaria follicular, distributing in post-ovarian fields. Eggs small, yellow, and 23–28 (25) by 11–14 (13).
Adult heterophyid flukes recovered from experimental cats and hamsters 8–10 days after infection. (A) H. taichui: body small, 591×298 in average size, with a muscular oral sucker (OS) and pharynx (P), a ventrogenital sac (VGS) with 11–18 rodlets, a saccular seminal vesicle (SV), a spherical ovary (O), single globular testis (T), and follicular vitellaria. (B) H. pumilio: body small, 485×223 in average size, with an oral sucker (OS), pharynx (P), a small ventrogenital sac (VGS) with 36–42 deer horn-like minute spines, a saccular seminal vesicle (SV), a spherical ovary (O), single globular testis (T), and follicular vitellaria. (C) Procerovum sp. (ventral view): body small, 435×238 in average size, with an oral sucker (OS), pharynx (P), a small ventral sucker, a long and thin-walled expulsor (E) and another seminal vesicle (SV), a spherical ovary (O), single globular testis (T), and follicular vitellaria. (D) Procerovum sp. (dorsal view). (E) C. formosanus (normal type): body very small, 389×196 in average size, with an oral sucker (OS) armed with about 32 circumoral spines, a muscular pharynx (P), a well-developed ventral sucker (VS), a spherical ovary (O), 2 globular testes (T), and follicular vitellaria distributing along extracecal margins from the pharyngeal level to the posterior end. (F) C. formosanus (plump type). (G) S. falcatus: body small, 447×233 in average size, with a muscular oral sucker (OS) and pharynx (P), a small ventral sucker and a long and thick-walled expulsor (E), a spherical ovary (O), 2 globular testes (T), and follicular vitellaria. (H) P. cambodiensis: body small, 467×277 in average size, with a muscular oral sucker (OS) and pharynx (P), a pair of eyespots, a small ventral sucker (VS), a ventrogenital complex (VGC) with a large genital sac and 2 gonotyls, a spherical ovary (O), 2 globular testes (T), and follicular vitellaria. All scale bar is 100 μm.
H. pumilio (n=20, Fig. 2B): Body small, pear-shaped, 400–600 (485) long, and 200–260 (223) wide, with greatest width at middle, the ovarian level. Oral sucker subterminal, 43–53 (45) by 50–63 (55). Pharynx subglobular or elliptical, 30–38 (33) by 18–30 (21). Esophagus short, 50–113 (73) in length. Ventrogenital sac small with 36–42 deer horn-like minute spines, 48–75 (62) by 30–63 (47). Seminal vesicle saccular. Ovary spherical or subspherical, 40–68 (56) by 50–80 (61), slightly dextral to midline. Seminal receptacle elliptical, lying the right-side of ovary. One testis globular or subglobular, 65–113 (90) by 75–113 (97), lying posterior 1/4 of body. Uterus with eggs occupying from anterior 1/3 to posterior end, most of hind-body. Vitellaria follicular, distributing in post-ovarian fields. Eggs small, yellow, and 28–33 (30) by 14–17 (15).
Procerovum sp. (n=3, Fig. 2C, D) Body small, pear-shaped, 425–445 (435) long, and 230–245 (238) wide, with greatest width at mid-level, between the ovary and testis. Oral sucker subterminal, 38–43 (40) by 40–45 (42). Pharynx subglobular or elliptical, 25–30 (28) by 20–25 (23). Esophagus short, 50–53 (51) in length. Ventral sucker very small, 18–20 (19) by 20–23 (21), embedded in ventrogenital sac. Seminal vesicle bipartite with thin-walled chambers, long expulsor, 118–168 (148) by 18–20 (19), and saccular portion, 88–95 (90) by 25–33 (28). Ovary spherical or subspherical, 40–65 (54) by 63–75 (71), slightly dextral to midline. Seminal receptacle saccular, 45–83 (63) by 33–55 (44), lying the right-side of testis. One testis globular or subglobular, 155–163 (160) by 115–163 (147), situated in middle of hind-body. Uterus with eggs occupying from anterior 1/3 to posterior end, most of hind-body. Vitellaria follicular, distributing from posterior border of ovary to posterior extremity. Eggs small, yellow, and 25–28 (26) by 13–15 (14).
C. formosanus (n=20, Fig. 2E): Body very small, 310–470 (389) long, and 150–220 (196) wide. Oral sucker subterminal, 38–55 (46) by 43–55 (50), armed with about 32 circumoral spines. Prepharynx very short. Pharynx globular, 30–45 (37) by 20–38 (30). Esophagus very short, 18–35 (24). Ventral sucker round or elliptical, 30–53 (42) by 40–60 (50). Ovary elliptical, 30–63 (45) by 43–80 (64), dextral to midline. Seminal receptacle large and saccular, 38–75 (51) by 30–75 (48). Two testes ellipsoidal, side by side near the posterior end; right 25–55 (46) by 63–93 (79), left 25–53 (43) by 55–83 (68). Vitellaria follicular, distributing along extracecal margins from pharyngeal level to posterior end. Eggs small, yellow, and 31–35 (34) by 16–19 (18). The morphological characteristics of normal type were nearly equal to those of plump type (Fig. 2F) except for the ratio of BL (body length) to BW (body width) (Table 6).

Dimensions of Centrocestus formosanus adults recovered in the small intestines of cats experimentally infected with metacercariae from Myanmar fish
S. falcatus (n=10; Fig. 2G) were small, oval to pyriform, dorsoventrally flat, 400–515 (447 in average) long and 215–245 (233) wide. Oral sucker subterminal, 35–42 (40) by 40–52 (47). Pharynx subglobular, 24–32 (28) by 15–28 (22). Esophagus slender, 45–65 (58) long. Ventral sucker small. Expulsor long and thick-walled, 103–135 (117) by 28–36 (33). Seminal vesicle saccate. Ovary spherical, 52–73 (57) by 55–88 (67). Two testes ovoid or globular, slightly oblique and widely separated; right 104–135 (115) by 68–82 (75); left 100–130 (114) by 57–75 (66). Vitellaria follicular, distributing in the post-ovarian fields. Eggs small, yellow, and 22–24 (23) by 11–13 (12).
P. cambodiensis (n=3, Fig. 2H): Body small, tapering anteriorly and bluntly ending posteriorly, 395–530 (467) long and 230–350 (277) wide, with a deep ventral concave. Eye-spot pigment seen in 2 lateral fields of anterior body. Oral sucker small, subterminal, subglobular, 45–48 (46) by 43–45 (43). Pharynx muscular, oval or slightly elongated, 33–38 (34) by 20–25 (23). Esophagus slender, 45–65 (58) in length. Ventral sucker small, globular, median, almost equal in size with oral sucker, 40–45 (42) by 45–50 (48). Ventrogenital complex well developed with large genital sac and 2 gonotyls. Ovary spherical, anterior to right testis, 55–68 (62) by 58–80 (68). Testes 2, separated, side-by-side, oval, transversely elongated, near the posterior body wall, right 48–60 (53) by 80–100 (87), left 48–63 (55) by 88–95 (92). Seminal vesicle bipartite, globular or transversely elongate, filled with sperms, in the mid field of body. Seminal receptacle elongates saccular, posterodorsal to ovary, 95–100 (97) by 50–55 (52). Gonotyls 2, sinistral to ventral sucker, round to elliptical, equipped with chitinous rodlets in 2 rows. Vitellaria follicular, distributing in post-ovarian fields, almost down to the posterior end of body. Eggs ovoid to elliptical, operculate, yellow, and 21–23 (22) by 10–13 (11).
DISCUSSION
Although the number and species of fish examined in this study were not so much, more than 7 species of ZT metacercariae (H. taichui, H. pumilio, H. yokogawai, S. falcatus, P. cambodiensis, Procerovum sp., and Centrocestus spp. including C. formosanus) were detected in fish from a local market of Yangon City, Myanmar. They were all minute intestinal flukes and members of the Heterophyidae. These heterophyid species have never been reported in Myanmar. Therefore, by the present study, it has been first confirmed that the life cycles of more than 7 species of heterophyid flukes are existing around Yangon, Myanmar.
Approximately 48 fish species have been reported as the second intermediate hosts of H. taichui in Asian countries, i.e., India, China, the Philippines, Thailand, Vietnam, and Lao PDR [18–27]. In the present study, H. taichui metacercariae were detected in 58 (22.0%) fish in 5 (41.7%) species, i.e., T. thynnoides, P. aurotaeniatus, E. altus, Mystacoleucus sp. and Labeo sp., and their density was 42.9 per fish infected. The infection rates and densities were relatively low when compared with those of previous studies performed in other countries [23–27]. The metacercariae of H. taichui detected in fish from Myanmar in this study (203×172 μm in average) were nearly the same in size with those from Vietnam (205×175) [25] and China (203×168) [24]. However, adult flukes of H. taichui in this study (591×298) were somewhat smaller than those of Chai et al. (655×305: 8-day-old in hamster) [25] and Dung et al. (756×421: recovered from residents in Nam Dinh Province, Vietnam) [7].
As the second intermediate host for H. pumilio, total 39 fish species have been recorded in China, Vietnam, and Cambodia [25,26,28]. In the present study, H. pumilio metacercariae were detected in 96 (36.4%) fish belonging to 9 (75.0%) species, i.e., T. thynnoides, P. aurotaeniatus, E. altus, C. striata, A. testudineus, T. pectoralis, Rhynogobius sp., Mystacoleucus sp., and Labeo sp., out of 264 examined fish which belong to 12 species, and their density was 23.6 per fish infected. Therefore, by the present study, 7 more fish species, i.e., T. thynnoides, P. aurotaeniatus, E. altus, C. striata, A. testudineus, and T. pectoralis, have been newly added as the fish intermediate hosts for H. pumilio. The metacercariae of H. pumilio in this study (176×160 μm in average) were nearly the same in size with those from Vietnam (179×159) [25] and China (172×152) [24]. Adult flukes in this study (485×223) were also nearly the same in size with those of Chai et al. (496×217) [25], but they were somewhat smaller than those of Dung et al. (632×291: recovered from residents in Nam Dinh Province, Vietnam) [7].
The metacercariae of H. yokogawai were reported in Asian countries such as India, Thailand, Egypt, Lao PDR, and Cambodia. They were found in about 43 fish species in these countries [4,18,20,21,23,28]. In the present study, H. yokogawai metacercariae were found in 5 fish species, i.e., P. aurotaeniatus, E. altus, T. pectoralis, Mystacoleucus sp., and Labeo sp., from Yangon, Myanmar. Among these fish, 2 species, P. aurotaeniatus and Mystacoleucus sp., were heavily infected with H. yokogawai metacercariae. The size of these metacercariae (175–225×163–218) were nearly the same as those of Rim et al. (170–240×150–230) from Lao PDR [23]. However, we failed to obtain the adult flukes from a cat and hamsters experimentally infected with the metacercariae.
Centrocestus formosanus has been distributed in China, Taiwan, Japan, Philippines, India, Lao PDR, Vietnam, and Cambodia [18,19,22–26,28,31–34]. The metacercariae of this fluke were detected in 9 fish species from Lao PDR [22,23,26,29,33, 34], in 16 species from Vietnam [8,25], in 10 species from Zhuang Autonomous Region, China [24], and in 2 fish species from Cambodia [28]. In the present study, Centrocestus spp. metacercariae including C. formosanus were detected in 8 (66.7%) fish species, i.e., T. thynnoides, P. aurotaeniatus, E. altus, C. striata, A. testudineus, T. pectoralis, Mystacoleucus sp., and Labeo sp., and their density was 25.8 per fish infected. The size of C. formosanus metacercariae in this study (171×142) were nearly the same with those from Vietnam (169×138) [25] and Lao PDR (150–200×100–120, and 180×107) [23,33,34], but somewhat smaller than those from China (208×164) [24]. Adult flukes in this study (389×196) were also nearly the same in size with those from Vietnam (367×207) [25], but they were somewhat smaller than those of Chai et al. (460×180: recovered from residents in Xiengkhouang Province, Lao PDR) [34].
In the present study, Procerovum sp. metacercariae were detected only in the climbing perch, Anabas testudineus, examined in June 2015. These fish were previously reported as the second intermediate host of Procerovum varium in Vietnam and Lao PDR [8,25,29,35]. The metacercariae of Procerovum varium were found in 100%, 30.0%, and 37.5% of the climbing perch from Hanoi City and Nam Dinh Province in Vietnam and Vientiane Municipality in Lao PDR, and their mean densities were 466, 13.5, and 12.7 per fish infected, respectively [25,29]. Whereas, Procerovum sp. metacercariae were found in 50.0% of the climbing perch from Yangon in Myanmar, and the density was 46.7 per fish infected in this study. Additionally, 9 fish species, i.e., L. rohita, H. molitrix, C. mrigala, C. idella, S. curriculus, P. brachypomum, C. batrachus, B. gonionotus, and M. cephalus, were recorded as the fish intermediate hosts of P. varium in Vietnam and Lao PDR [8,25,29,35].
Procerovum sp. metacercariae (185×155) detected in this study were similar in size with P. varium metacercariae (187×147) in the climbing perch from Hanoi City [25], Vietnam, but they were slightly larger than the P. varium metacercariae (175×136) in same fish hosts from Vientiane Municipality in Lao PDR [29]. However, their general morphologies, i.e., elliptical in shape, presence of brownish pigment granules in the worm body, a prominent expulsor, and a D-shaped excretory bladder with grouped excretory granules, were almost identical to those of the previous studies [25,29].
Trematode members in the genus Procerovum (Family Heterophyidae) are characterized by possessing a single testis and a long prominent seminal vesicle modified into an expulsor. Among the 5 species listed, 3 species, i.e., P. varium, P. calderoni, and P. cheni, have been certified for their validity based on the morphology of the seminal vesicle. Our specimens (435×238) were similar in size with those of Chai et al. [25] (434×223), which were recovered from a hamster experimentally infected with the metacercariae from fish of Vietnam, but they were somewhat larger than those of Eom et al. [29] (304×186), which were recovered from a cat experimentally infected with the metacercariae from fish of Lao PDR. The length of expulsor (148 μm) is more or less longer than that in Chai et al. [25] (115) and Eom et al. [29] (123). Moreover, the expulsor in our specimens has a bipartite seminal vesicle with thin-walled chambers like that in P. cheni. However, the expulsor is saccular and thick-walled in P. varium and P. calderoni. Collectively, the length of expulsor is less than 100 μm in P. cheni, less than 160 μm in P. varium, and more than 200 μm in P. calderoni [25,36]. The morphological characteristics of the expulsor in our specimens are mixed with those of P. varium and P. cheni. It is seriously needed to clarify the valid species name of Procerovum sp. with further precise studies in Myanmar.
The metacercariae of S. falcatus were detected only in the mullet, Chelon macrolepis, like in Cambodia [37]. They were also found in several species of fish, i.e., mullet (M. cephalus and L. haematocheila), goby (A. flavimanus), wrestling halfbeak (D. pusilla), X. canciloides, giant gouramy (O. gourami), common carp (C. carpio), and grass carp (C. idella), in Japan, Hawaii, China, Korea, Thailand, Vietnam, and Lao PDR [38–45]. Among these fish intermediate hosts, the mullet is the most frequently reported in various regions [25,37–41]. The size of S. falcatus metacercariae in this study (225×165) were nearly the same with those in Cambodia (220×168), but somewhat smaller than those in Vietnam (297×232) [25,37]. The adult worms (447×233) were nearly the same in size with those of Chai et al. (450×237: 10-day-old in a hamster), but they were slightly smaller than those of Dung et al. (468×298: from residents of Vietnam) and Chai et al. (481×239: 8-day-old in a hamster) [8,25].
The metacercariae of P. cambodiensis were found only in the mullet, Chelon macrolepis, like in a previous study, and their size (242×194) and morphology were nearly equal to those of Sohn et al. (246×191) from Cambodia [46]. In the adult worms, the size of our specimens (467×277) were smaller or less than those of Sohn et al. (534×306: 10-day-old in a hamster). However, other morphological characteristics, i.e., distribution of vitellaria and uterus, and genital organs, were closely identical with those of P. cambodiensis n. sp. previously described by Sohn et al. [46]. In particular, the morphology of ventrogenital complex, i.e., a muscular, sucker-like genital sac equipped with 2 plate-like gonotyls, which are armed with chitinous rodlets (4–5 on the right side and 10–11 on the left side in 2 rows), is a species-specific character of P. cambodiensis.
Conclusively, it has been first confirmed in Myanmar that more than 7 ZT species, i.e., H. taichui, H. pumilio, H. yokogawai, S. falcatus, P. cambodiensis, Procerovum sp., and Centrocestus spp. including C. formosanus, of heterophyid flukes are distributed around Yangon area, and some species of fish play the role of second intermediate hosts for these trematodes. Although there are some drawbacks in this study, such as the obscure collection sites, the small number and species of fish, and a long survey period, epidemiological trends of ZT infections in Myanmar have fairly been revealed.
ACKNOWLEDGMENTS
We thank Jung-A Kim and Hee-Ju Kim, Department of Parasitology, Gyeongsang National University College of Medicine, Jinju, Korea, for their help in fish examinations. We also thank the staff of the Korea Association of Health Promotion, Seoul, the Republic of Korea, who participated in the Korea-Myanmar Cooperation Project on Parasite Control in Myanmar (2013–2015).
Notes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest with this study.