Leishmania tropica infection, in comparison to Leishmania major, induces lower delayed type hypersensitivity in BALB/c mice
Article information
Abstract
Leishmania tropica and L. major are etiologic agents of human cutaneous leishmaniasis. Delayed type hypersensitivity (DTH) is an immunologic response that has been frequently used as a correlate for protection against or sensitization to leishmania antigen. In BALB/c mice, L. tropica infection results in non-ulcerating disease, whereas L. major infection results in destructive lesions. In order to clarify the immunologic mechanisms of these 2 different outcomes, we compared the ability of these 2 leishmania species in induction of DTH response in this murine model. BALB/c mice were infected with L. major or L. tropica, and disease evolution and DTH responses were determined. The results show that the primary L. major infection can exacerbate the secondary L. major infection and is associated with DTH response. Higher doses of the primary L. major infection result in more disease exacerbation of the secondary L. major infection as well as higher DTH response. L. tropica infection induces lower DTH responses than L. major. We have previously reported that the primary L. tropica infection induces partial protection against the secondary L. major infection in BALB/c mice. Induction of lower DTH response by L. tropica suggests that the protection induced against L. major by prior L. tropica infection may be due to suppression of DTH response.
INTRODUCTION
Leishmania tropica and L. major are etiologic agents of human cutaneous leishmaniasis. Although these 2 leishmania species share in many common characters (Mahmoudzadeh-Niknam and McKerrow, 2004), there are some differences between the clinical patterns of disease caused by these 2 species. Murine models can help elucidation of the different immunopathological mechanisms of the disease caused by these leishmania species. L. tropica and L. major infection of BALB/c mice result in 2 different outcomes. L. tropica infection in BALB/c is not fatal and results in chronic, non-healing, and non-ulcerating disease (Lira et al., 1998). However, L. major infection in BALB/c results in progressive non-healing and destructive lesions (Lira et al., 1998).
Delayed type hypersensitivity (DTH) is an immunologic response that has been frequently used as a correlate for protection against or sensitization to leishmania antigen in humans and experimental models of leishmania infection (De Rossell et al., 1987, Liew and Dhaliwal, 1987, Dhaliwal and Liew, 1987, Convit et al., 1993). In order to clarify the immunologic mechanisms underlying different clinical picture of L. tropica and L. major infection in BALB/c mice, we studied and compared the ability of these 2 leishmania species in induction of DTH response in this murine model.
Materials and Methods
Mice
Inbred female BALB/c mice, 6-8 week old, were used throughout the experiments. These mice were obtained from the animal breeding facility of Pasteur Institute of Iran.
Parasites
Leishmania tropica strain MHOM/AF/88/KK27 is a cutaneous type isolated from Afghanistan, and was initially described by Dr. R. Killick-Kendrick (Lira et al., 1998). It was a gift from Dr. D. Sacks (Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Disease, National Institute of Health, Bethesda, Maryland, USA). The L. major strain MRHO/IR/75/ ER is an isolate from Iran, and was a gift from Dr. M. Mohebali (School of Public Health, Tehran University of Medical Sciences, Tehran, Iran). The parasites were grown at 23-24℃ in 50 ml disposable centrifuge tubes containing 4 ml of culture medium. The culture medium consisted of 2 ml NNN and 2 ml RPMI-1640 medium (Sigma Chemical Co, St Louis, Missouri, USA). The NNN medium consisted of 12% rabbit blood (with sodium citrate as anticoagulant), 1.35% glucose (Sigma Chemical Co), 1.4% agar (Merck, Darmstadt, Germany), and 0.6% (w/v) NaCl (Arasto Pharmaceutical Chemicals Co, Saveh, Iran). Penicillin (100 IU/ml) and Streptomycin (100 µg/ml) were used as antibiotics.
Infection
Mice were infected intradermally by defined doses of live stationary phase promastigotes in the ear dermis for a primary infection and in the hind footpad for a secondary infection. The disease evolution was monitored by measurement of thickness of ear or footpad at weekly intervals. The thickness was deterMice were infected intradermally by defined doses of live stationary phase promastigotes in the ear dermis for a primary infection and in the hind footpad for a secondary infection. The disease evolution was monitored by measurement of thickness of ear or footpad at weekly intervals. The thickness was determined by a dial-gauge caliper (Mitutoyu, Kawasaki, Kanagawa, Japan).
Delayed type hypersensitivity (DTH)
DTH responses to the secondary infection were determined by measuring footpad thickness at 18-24, 48, and 72 hr post-secondary infection. DTH values were calculated as the thickness of parasite-injected footpad minus thickness of uninjected contralateral footpad.
Study design
BALB/c mice, in groups of 5-10 mice, were infected in the ear by L. major or L. tropica as a primary infection, and disease evolution was monitored by determination of the ear thickness. A secondary infection was done by injection of L. major or L. tropica in the hind footpad, 2-4 mo after the primary infection. DTH responses to the secondary infection were determined by determination of footpad thickness at defined times after the secondary challenge. Each experiment was repeated at least once, and data from one representative experiment is shown.
Statistical analysis
Footpads or ear thicknesses were compared between different experimental groups by Student's t-test. P-values, equal to or less than 0.05, were considered significant.
RESULTS
Higher doses of L. major infection result in earlier appearance of lesion and higher ear thickness increase in BALB/c mice
BALB/c mice were infected in the ear with 103, 104, 105 stationary phase L. major promastigotes. Ear thickness was determined up to 20 weeks after infection. Results show that higher doses of L. major results in earlier appearance of the lesion and higher ear thickness (Fig. 1). The ear thickness differences were statistically significant between all 3 groups at week 8 and 10 after infection (P < 0.008 and P < 0.05, respectively). Injection of 103 dose of L. major was able to establish infection as evidenced by increase of ear thickness. Our data shows that, although infection by all doses finally results in lesion development, the development of lesion was significantly delayed in lower dose infections. In other words, when we compared the state of disease exacerbation in mice infected with different doses of parasites at a definite time after a primary infection (for example at the time of a secondary infection which was 2 mo after the primary infection), the disease was already exacerbated in mice infected by 105 parasites, while lesion did not even appear in mice infected by 103 parasites (see below). It means that mice received a secondary L. major infection at different states of disease exacerbation originated from different doses of primary infection.
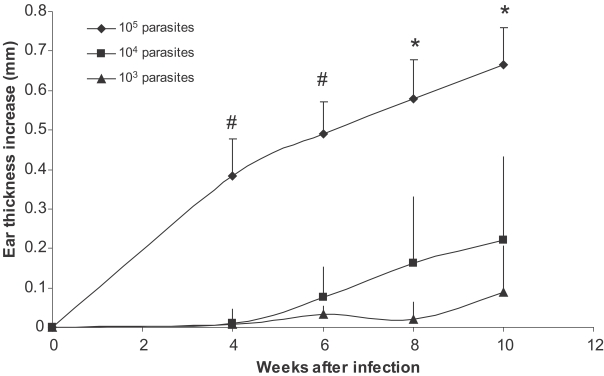
Effect of parasite dose of L. major infection on ear thickness in BALB/c mice. L. major at doses of 105, 104, and 103 were injected into the ear intradermally. Ear thickness increase was defined as thickness of infected ear minus thickness of contralateral uninfected ear. Data are mean + SD of 5-10 mice per group. Symbol "#" shows statistically significant differences (P < 0.05) between doses of 105 and 104. Symbol "*" shows statistically significant differences (P < 0.05) between all the three doses (105, 104, and 103).
Primary L. major infection in BALB/c mice exacerbated secondary L. major infection and is associated with early DTH response
BALB/c mice were infected by injection with 105 stationary phase L. major promastigotes intradermally into the ear dermis. One million stationary phase L. major promastigotes were injected subcutaneously into hind footpad 2-3 mo after a primary infection, and DTH responses were determined up to 3 days and footpad thickness determined for up to 20 weeks. The results are shown in Figs. 2 and 3. It was shown that primary L. major infection can; (1) exacerbate secondary L. major infection in BALB/c mice in a statistically significant manner (P < 0.001 in all compared intervals), and (2) induce higher DTH responses at 24 hr post-injection (P < 0.0001). So, DTH is associated with more exacerbation of a secondary L. major infection in BALB/c mice in our experimental system.

Effect of primary L. major infection on secondary L. major infection of BALB/c mice. Mice were infected by 105 L. major in the ear as the primary infection followed by 106 L. major in footpad as the secondary infection. The secondary infection was done 2-3 mo after the primary infection. Footpad thickness increase was defined as thickness of infected footpad minus thickness of contralateral uninfected footpad. Data are mean + SD of footpad increase of 5-10 mice per group after the secondary infection. Asterisk (*) shows statistically significant difference (P < 0.05) between the 2 groups.

Effect of primary L. major infection on delayed type hypersensitivity (DTH) response to secondary L. major infection in BALB/ mice. Mice received 105 L. major in the ear as the primary infection followed 2-3 mo later by 106 L. major in the footpad as the secondary infection. DTH were determined in the footpad after the secondary infection. Footpad thickness increase was defined as thickness of infected footpad minus thickness of contralateral uninfected footpad. Data are footpad increase of individual mice.
Higher doses of primary L. major infection induce more disease exacerbation of secondary L. major infection and are associated with higher DTH responses in BALB/c mice
Experiments were carried out for study on the effect of the dose of primary L. major primary infection on exacerbation of the secondary L. major infection as well as on DTH responses to this secondary infection. Primary infections were done by 103, 104, or 105 doses of stationary L. major in ear dermis. The secondary infection was done by 106 stationary L. major promastigotes in footpad, 2-3 mo after the primary infection. Disease evolution and DTH response of these experiments are shown in Figs. 4 and 5. Footpad thickness increased more rapidly in mice infected with higher doses of primary L. major infection (P < 0.05 between all 3 groups at week 12 after secondary infection). DTH responses at 24 hr after the secondary infection were also higher in mice receiving higher doses of L. major as the primary infection (P < 0.05 between mice receiving different doses of the primary infection). Based on these results, and for excluding the effect of infecting dose, we decided to compare the same infectious dose of L. major and L. tropica for their DTH induction capability in BALB/c mice.
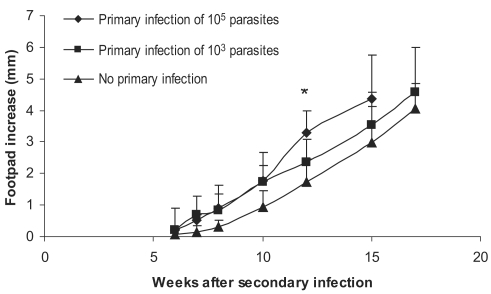
Effect of dose of primary L. major infection on secondary L. major infection in BALB/c mice. Mice received the primary infection of 105 or 103 L. major in the ear followed by 106 L. major in footpad as the secondary infection 2-3 mo later. Footpad thickness increase was defined as thickness of infected footpad minus thickness of contralateral uninfected footpad. Data are mean + SD of footpad increase of 5-10 mice per group. Asterisk (*) shows statistically significant difference (P < 0.05) between the 3 groups.

Effect of dose of primary L. major infection on delayed type hypersensitivity (DTH) response of BALB/c mice to secondary L. major infection. Mice received 105, 104, and 103 doses of L. major in the ear as the primary infection followed by 106 L. major in footpad as the secondary infection 2-3 mo later. Footpad thickness increase was defined as thickness of infected footpad minus thickness of contralateral uninfected footpad. Data are mean + SD of footpad increase of 5-10 mice per group after the secondary infection. Asterisk (*) shows statistically significant difference (P < 0.05) between doses of 105, 104, and 103.
Lower DTH responses by L. tropica in comparison to L. major in BALB/c mice
Mice were infected with 105 stationary phase promastigotes of L. major or L. tropica into ear dermis as the primary infection. Mice received 106 stationary phase promastigotes of L. major or L. tropica as the secondary infection into hind footpad about 3-4 mo after the primary infection. DTH responses against the secondary infection were determined at 18-24, 48, and 72 hr post-secondary infection. As shown in Fig. 6, the DTH response against L. major was statistically higher in L. major-infected mice in comparison to L. tropica-infected mice (P < 0.0001). Similar statistically important differences were observed between the DTH response against L. tropica secondary infection in mice that received L. major or L. tropica as the primary infection (P < 0.0001).

Effect of primary infection of L. major or L. tropia on delayed hypersensitivity (DTH) response against secondary L. major or L. tropica infection in BALB/c mice. Mice received 105 L. major or L. tropica in the ear as the primary infection followed by the secondary infection of 106 L. major or L. tropica in footpad 3-4 mo later. Footpad thickness increase was defined as thickness of infected footpad minus thickness of contralateral uninfected footpad. Data are mean + SD of footpad increase of 5-10 mice per group 18-24 hr after the secondary infection. Asterisk (*) shows statistically significant difference (P < 0.05) between the groups inside the brackets.
DISCUSSION
The effect of the primary L. major infection on the secondary L. major infection has been reported with conflicting results. Babay et al. (2004) reported that lesion development of the secondary L. major infection is not significantly different from the primary L. major infection in BALB/c mice. Compton and Farrell (2002) reported that a low dose (250 parasites) of L. major results in a progressive lesion and does not result in protection against the disease. There are some reports that a primary low-dose L. major infection can protect BALB/c mice against a secondary L. major challenge (Bretscher et al., 1992; Doherty and Coffman, 1996, Courret et al., 2003). Courret et al. (2003) reported that a primary low dose (10-1,000 parasites) of L. major infection induces protection against a secondary challenge of 1,000 metacyclic L. major. Our results (Fig. 4) show that a primary low dose (1,000) of L. major infection did not induce protection against a secondary challenge of 106 stationary phase promastigotes. The discrepancy of our results with others may be due to different experimental conditions, including the different dose or growth phase of the parasite used for infection or challenge and also use of different parasite strains.
DTH response in BALB/c mice has been extensively studied (De Rossell et al., 1987). DTH response has 2 phases in BALB/c mice; early and late. The early DTH response peaks at 15 hr after injection of leishmania antigen (Liew and Dhaliwal, 1987). This DTH response has characteristics of Jones-mote reaction (De Rossell et al., 1987; Liew and Dhaliwal, 1987). The late phase DTH response peaks at 24-48 hr after injection of leishmania antigen and has the characteristics of tuberculin-type DTH (De Rossell et al., 1987; Liew and Dhaliwal, 1987). Dhaliwal and Liew (1987) reported that the early appearing type of DTH is not only dissociable from protective immunity but also can facilitate the development of cutaneous leishmaniasis. Our results are compatible with this report and confirm that enhancement of early DTH response is associated with disease exacerbation in BALB/c model of L. major infection. In agreement with our findings regarding anti-protective role of DTH, Liew et al. (1985) reported that transfer of DTH by T cell-enriched spleen cells can abrogate protection in BALB/c mice.
Our results clearly show that L. tropica infection in BALB/c mice induces significantly lower early DTH response, comparing to the DTH induced by L. major infection in the same mouse strain. The effect of parasite dose on the observed differences of DTH responses is not likely in our study, because we carefully determined the dose and used the same dose for L. major and L. tropica in our experiments. The possible effect of the quality of eliciting antigen (used for the secondary challenge) on the DTH response is also ruled out, because the lower DTH induced by L. tropica in comparison to L. major were shown with use of either L. major or L. tropica as eliciting antigens.
It has been shown that primary L. tropica infection of BALB/c mice can partially protect secondary L. major challenge of the same mice (Mahmoudzadeh-Niknam 2004). The findings in this study suggest that this protection may be mediated by immunological mechanisms resulting in low early DTH response. Early DTH response in BALB/c mice has been shown to be mainly due to eosinophils and basophilic mast cells (De Rossell et al., 1987). Mast cells play a pro-parasitic role in BALB/c mice (Saha et al., 2004) and eosinophils are effector cells of T-helper-2 (Th2) responses (Abbas and Litchman, 2004). So, reduction of early DTH response by prior L. tropica infection may originate from low activity of Th2 responses. If this conclusion is valid, L. tropica may be able to induce some suppression mechanisms, which results in lower Th2 responses.
Our findings suggest that protection induced by L. tropica may be due to suppression of DTH response. In agreement with our findings, the presence of specific suppressor T cells for DTH has already been reported in L. major-infected BALB/c mice (Howard et al., 1980; Dhaliwal et al., 1985). Potential role of L. tropica infection in suppression or regulation of Th2 response in BALB/c mice needs more experiments and is under investigation in our laboratory.
Most cases of normal cutaneous leishmaniasis consist of self-healing skin lesions that are associated with strong specific DTH. However, the skin-test reactivity is absent in non-healing diffuse cutaneous leishmaniasis. On the contrary, DTH is exaggerated in an allergic non-healing form of cutaneous leishmaniasis, lupoid leishmaniasis or leishmania recidivance (De Rossell et al., 1987). Exaggerated DTH is also seen in some forms of american cutaneous leishmaniasis, which produce mucosal lesions (Convit et al., 1993). Presence of high early DTH response in L. major infected BALB/c mice and exacerbated DTH response in human lupoid leishmaniasis suggest that these 2 non-healing leishmaniasis types may have common immunologic basis. So, immunologic mechanisms responsible for suppression of DTH response in BALB/c mice may have relevance for study of DTH-positive non-healing cases of human leishmaniasis.
In summary, our data shows that L. tropica infection in BALB/c mice is associated with low DTH response. The relevancy of this low DTH response to the protective immunity in this murine model needs more study.
ACKNOWLEDGMENTS
Kind advice from Dr. David Sacks (Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Disease, National Institute of Health, Bethesda, Maryland, USA) in design and execution of the experiments are highly appreciated. Critical reading of the manuscript by Dr. David Sacks and Dr. Mary Wilson (University of Iowa, Iowa City, Iowa, USA) are appreciated. We are thankful to Dr. M. H. Alimohammadian (Immunology Department of Pasteur Institute of Iran) for managerial support.
References
Notes
This research received financial supports from Pasteur Institute of Iran (Research Project No. 275).