Changes of cytokine mRNA expression and IgG responses in rats infected with Capillaria hepatica
Article information
Abstract
The mRNA expression of several cytokines was evaluated in splenocytes and mesenteric lymph node (MLN) cells of rats infected with Capillaria hepatica by reverse-transcription (RT)-PCR until week 12 after infection. IgG1 and IgG2a, which are associated with Th1 and Th2 response, respectively, were also assessed by ELISA. The results indicated that the majority of cytokines, including the Th1 (IL-2 and IFN-γ) and Th2 cytokines (IL-4, IL-5 and IL-10) were expressed at maximal levels during the early stage of infection (after week 1-2), and the ELISA data also evidenced a similar pattern of changes in IgG1 and IgG2a. Th1 and Th2 cytokines responded in a similar fashion in this rat model. The expression of cytokines in splenocytes was significantly higher than that in MLN cells, thereby indicating that cytokine production is controlled more by spleen than by MLN. In addition, the observation that IFN-γ expression increased unexpectedly at the time of maximal egg production (6 weeks after infection) indicated that IFN-γ is a cytokine reacting against egg production. However, increased IL-5 expression occurring in tandem with worm activity indicated that the activity of C. hepatica might be controlled by IL-5 expression.
INTRODUCTION
Capillaria hepatica is a zoonotic liver nematode of mammals of worldwide distribution, including house rats in Korea. In humans, infections are rarely found. Only 37 human cases of C. hepatica infections have been reported between 1924 and 1996 (Juncker-voss et al., 2000), with 1 case of them having been in Korea (Choe et al., 1993). When hosts ingest embryonated eggs of C. hepatica, the infection occurs. From approximately 6 hr onwards after hatching, the larvae penetrate the intestinal mucosa, enter the hepatic portal vein, and are carried to the liver (Leuttermoser, 1938). Larval maturation is swift, and female worms bearing ova may be seen 18-21 days after the ingestion of embryonated eggs by the host (Luttermoser, 1938; Wright 1961). Normal egg production increases rapidly from day 20 post-infection (PI) to its peak at day 40 PI, with no further egg production occurring after about day 70 PI (Lammler et al., 1974).
In response to pathogenic invasion, humoral, cellular, or both types of immune response may be activated. Several studies have demonstrated the importance of the function of T-helper (Th) lymphocytes in the regulation of immunity to parasitic infections (Capron, 1992; Mosmann and Sad, 1996). In BALB/c mice infected with Leishmania spp., protective immunity has been shown to be related to the Th1 response, whereas pathogenesis has been demonstrated to be related to the Th2 response (Scott et al., 1989). In murine schistosomiasis, it has been demonstrated that a Th2 response is involved in the development of chronic infections, whereas a Th1 response participates in protection against infections with Schistosoma mansoni (Pearce and Sher, 1991; Pearce et al., 1991; Pierrot et al., 1996). With regard to Trichuris muris, there is a strong host genetic influence on the cytokine profile, the response of resistant strains being Th2-dominated, whereas that of susceptible strains switching to a Th1 response (Else and Grencis, 1991; Else et al., 1992). Th-subset polarization after parasitic infection has been demonstrated in several systems and reflects the influence of the initial, local cytokine environment.
The principal objective of the present study was to determine mRNA expression levels in splenocytes and MLN cells of rats infected with C. hepatica. The mRNA levels of various cytokines were evaluated by reverse transcription-PCR (RT-PCR), and ELISA analyses of IgG1 and IgG2a, which are associated with Th1 and Th2 responses in rats, respectively, were also conducted.
MATERIALS AND METHODS
Parasites and preparation of embryonated eggs
The livers of house rats infected with C. hepatica were digested with pepsin-HCl at 37℃. Eggs of C. hepatica were isolated by repeated filtration and washing of digested liver tissues. The eggs were then cultured in 0.5% formalin solution at 30℃ until embryonation. The embryonated eggs were maintained at -20℃ until they could be employed in preparation of the egg antigen or counted for infection to rats.
Parasite infection and sera collection
Sprague-Dawley rats were orally infected with 5,000 embryonated eggs. The normal and infected rat sera were collected routinely by ophthalmic venous plexus puncture and maintained at -70℃.
Egg antigen preparation
Antigen was prepared from embryonated eggs of C. hepatica using the freeze homogenizing method. The homogenized eggs were centrifuged at 10,000 rpm for 60 min and supernatant was collected. This was concentrated with Centri-plus™ (Amicon, Beverly, Massachusetts, USA) and the antigen concentrations were determined.
IgG1 and IgG2a assay
Serum concentrations of C. hepatica-specific IgG1 and IgG2a were determined by ELISA (Engvall and Perlman, 1972). In brief, 100 µL of C. hepatica egg antigen at a protein concentration of 10 µL /mL in carbonated buffer was coated onto the wells of a ELISA plate (Corning, New York, USA) and incubated overnight at 4℃. After the plate was washed with 0.1% Tween-20 containing saline, each well was blocked with 200 µL of 0.2% gelatin for 90 min at 37℃. After washing, 100 µL of the serum samples diluted to 1: 50 were added and incubated for 90 min at 37℃. After washing, 100 µL of peroxidase-conjugated mouse anti-rat IgG1 or IgG2a (Zymed, San Francisco, California, USA) diluted to a concentration of 1: 1,000 was applied to each of the well and incubated for 90 min at 37℃. After washing, a mixture of substrate OPD (o-phenylenediamine) and H2O2 in substrate buffer was added to the wells and the absorbance was read at 490 nm with an ELISA reader (Spectra MAX 250, Sunnyvale, California, USA).
Collection of splenic and MLN lymphocytes
The spleen and MLN were removed from 3 normal rats and 5 infected rats on different days after infection and crushed in phosphate-buffered saline. The lymphocytes were collected by 50% and 70% Percoll (Sigma, St. Louis, Missouri, USA) gradient method using the suspended cells and maintained at -70℃ until RNA extraction.
RT-PCR (reverse transcription-polymerase chain reaction)
In order to isolate total RNA, lymphocytes acquired from both spleen and MLN on different days after infection were homogenized in Trizol (Gibco BRL, Grand Island, New York, USA) and the RNA was then extracted with chloroform and precipitated with isopropanol, respectively. After ethanol washing, the total extracted RNA was resuspended in DEPC (diethyl-pyro-carbonate) - treated H2O.
The extracted total RNA was reverse transcribed with Oligo d(T)15 primer using AMV-reverse transcriptase (Promega, Madison, Wisconsin, USA) at 37℃ for 1 hr. The resulting cDNA was subjected to polymerase chain reaction (PCR) for 30 cycles with respective primers designed from the sequences of five cytokines and GAPDH (glycer-aldehydes-3-phosphate-dehydrogenase). The thermal cycle profile was as follows: 1 min at 95℃ (denaturation step), 1 min at respective optimal temperature (annealing step), and 1 min at 72℃ (extension step) on a PTC 100 thermal cycler (MJ Research, Water Town, Massachusetts, USA). Primer sequences for amplification and the respective sizes of the PCR products of cytokines and GAPDH cDNAs are shown in Table 1. PCR products were analyzed by electrophoresis on agarose gels and visualized using ethidium bromide.
Visualized PCR products by ethidium bromide were analyzed by the intensity using densitometry of image analyzer (Eagle-eye, Stratagene, La Jolla, California, USA). It was then standardized for comparing with GAPDH expression. Results were expressed as the fold increase over control.
RESULTS
RT-PCR detection of cytokine mRNA
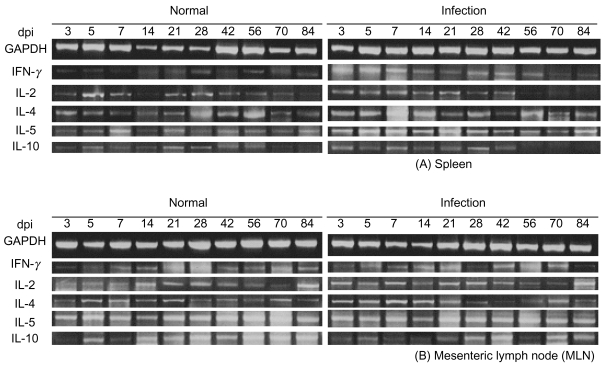
Sprague-Dawley rats were infected orally with 5,000 embryonated eggs per rat. The total RNA prepared from the collected spleens and MLNs was reverse transcribed. After repeated triple procedures, we determined the number of amplification cycles for each primer pair required for generation of appropriate hybridization signals. A standardized amplification protocol was established at 30 cycles, and the cytokine transcript levels were normalized to equal amounts of cDNA using the corresponding signals obtained for GAPDH RNA. The RT-PCR products are shown in Fig. 1. The results are presented as a fold increase over the control value for a given cytokine in a given organ.
Analysis of the Th1 and Th2 type cytokine mRNA
RNA was prepared from the spleens and MLNs of rats at different time points over the course of infection, as well as from uninfected control rats. Fig. 2 shows that IFN-γ expression in the spleen of infected rats was elevated at day 3 post-infection (PI) and reached maximum levels at day 7 PI. An increase of 6.5 fold in the IL-2 expression (Fig. 3) was observed in the spleen on day 7 PI. However, a less profound increase of IFN-γ and IL-2 was detected in the MLNs (Fig. 2).
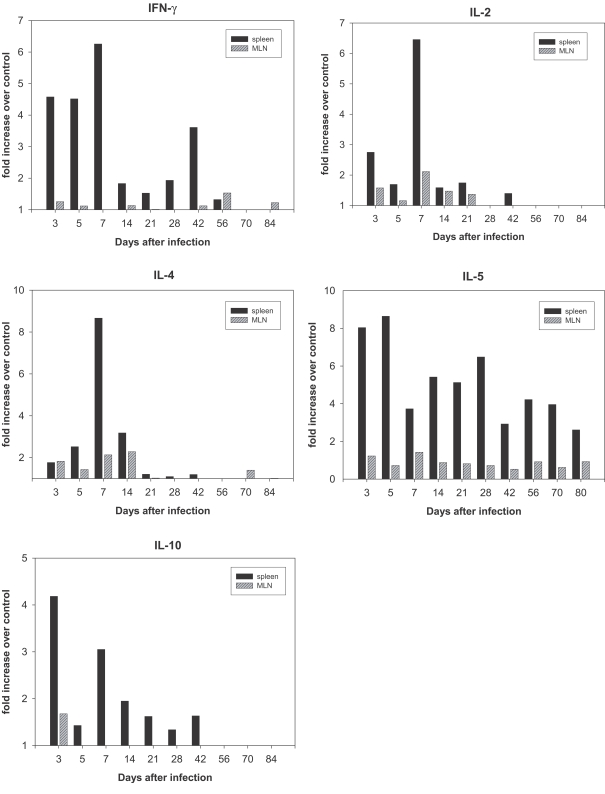
Expression of IFN-γ, IL-2, IL-4, IL-5, and IL-10 in rats infected with Capillaria hepatica. RNA was purified from the spleen and MLN for various infection times from infected and uninfected rats (control). Tissues were pooled from each group. Results from one representative experiment out of 3 kinetic studies are presented as fold increase.
In order to analyze the Th2 response during infection, we monitored the expression of IL-4, IL-5, and IL-10 mRNAs. IL-4 expression in the spleens of infected rats increased and reached a maximum increase of 8.6 fold at day 7 PI (Fig. 2), and the expression of IL-5 (Fig. 2) increased by 8.0-fold in the spleen on day 3 PI, and reached 8.7-fold increase at day 5 PI. IL-10 expression in the spleens increased maximal levels of 4.2- fold that of control levels on day 3 PI (Fig. 2). However, the increase in IL-5 and IL-10 mRNA expression in MLNs was not found to be significant (Fig. 2).
The majority of cytokines, including the Th1 cytokines (IL-2 and IFN-γ) and Th2 cytokines (IL-4, IL-5 and IL-10), were maximally expressed at the early stages of infection (after days 7-14) without Th1 and Th2 discrimination.
IgG1 and IgG2a antibody assay
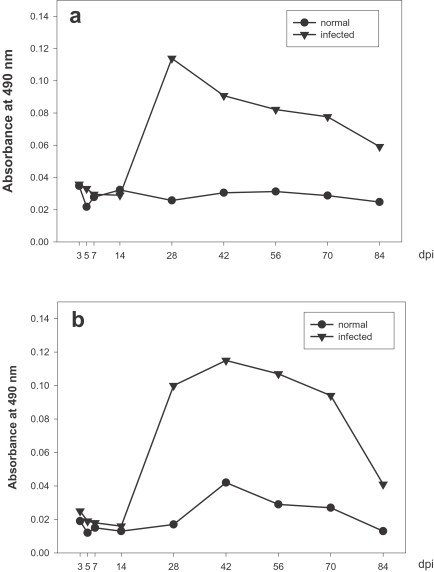
IgG1 titers were elevated at day 14 PI, rapidly reached maximum levels at day 28 PI, and then decreased gradually until the end of the study (Fig. 3a), and C. hepatica-specific serum IgG2a levels rose quickly at day 14 PI, increased gradually, reached a maximum at day 42 PI, and then decreased gradually until day 70 PI (Fig. 3b).
These ELISA data evidenced production patterns similar to those of IgG1 and IgG2a, thereby indicating that Th1 and Th2 cytokines respond similarly in the C. hepatica-infected rat model utilized in this study.
DISCUSSION
This is, to the best of our knowledge, the first study to focus on cytokine synthesis in rats infected with C. hepatica. In the case of parasitic infections, Th1 and Th2 cytokine responses vary with different infection sources or hosts (Pearce et al., 1991; Matsuda et al., 1995; Sugaya et al., 1997; Powell et al., 1998; Holland et al., 2000; Hoffmann et al., 2002; Maizels and Yazdanbakhsh, 2003; Maizels et al., 2004; Holland et al., 2005; Maizels, 2005). Dendritic cells are immune cells, and they form parts of the mammalian immune system, of which main function is to process antigens and induce Th type biased immune responses (MacDonald et al, 2001; Straw et al, 2003; Kane et al, 2004; McConchie et al., 2006; Pearce et al., 2006).
In rats infected with S. mansoni, a sudden increase in cytokine mRNA expression was observed only during the early stages of infection (day 7 PI), during which the parasite passes through the lung. This result indicates that the immune response is associated to the parasitic infection pathway (Cetre et al., 1998). C. hepatica is a parasite, which is largely unrelated to MLNs, parasitic in the liver parenchyma and requires only a short time to pass the intestine. Consequently, no changes in the cytokine expression were observed in MLN lymphocytes, and it was either increased or decreased to a significant degree in the spleen. Therefore, the immune response in the spleen, but not in the MLNs, may be involved in the control of cytokine secretion during the infection.
IFN-γ and IL-2, which are Th1 cytokines, and IL-4, IL-5 and IL-6, which are Th2 cytokines, play certain roles in immunity in vivo. However, the mechanisms by which these cytokines operate have been reported to vary widely. The mechanisms inherent to responses to foreign antigens differ depending on the kinds of parasites, host and infection period. When we set a standard with rates of increase or decrease in cytokine expression in the spleen, it was determined to increase principally in the early stages of infection, and it was unbiased toward the Th1 or Th2 cytokine. Furthermore, a significant increase in IL-2 and IL-4 expression was observed on day 7 PI. However, IL-5 expression levels increased immediately after infection, persisted at a high level until day 5 PI, and then decreased; however, these levels increased again during week 4 PI, when the worm had matured completely, and the levels evidenced a constant increasing trend during weeks 8-10 PI, during which the worm was extinct, thereby suggesting that IL-5 may be associated with worm development. In addition, IFN-γ levels increased during the early stage and then decreased; however, these levels became slightly increased again on week 6 PI, when egg expulsion peaked. Therefore, it appears quite likely that IL-5 is a cytokine that responds to egg expulsion.
It was difficult to determine the cytokine expression in MLNs as the expression level was generally low. Nevertheless, a slight increase was observed in MLN cytokine expression in the early stages of infection.
Immunological studies utilizing the sera of rats infected with C. hepatica have been conducted in several ways. The circumoval precipitation (COP) test was designed to diagnose infected sera via observation of the responses in a mucoid plug. However, the response according to the duration of infection was not observed (Kim et al., 1982). Until now, ELISA is the method most frequently used for this. Lee et al. (1985) reported that the total serum IgG levels in infected rats rise after 3 weeks, attaining maximal levels on weeks 3 and 5 PI and converting to negative at week 13 PI. We assessed the levels of antibodies secreted into the blood by cytokine via measurements of the antibody titer, using the IgG isotypes, IgG1 and IgG2a, and compared these levels to the levels of expressed cytokine.
It has been generally established that IgG2a production is promoted by IFN-γ and IgG1 production is promoted by IL-4. However, the responses in rats differ from those of mice or humans, and it has been reported that IgG1 production is promoted by IFN-γ and that IgG2a production is by IL-4 (Philips et al., 1999). Therefore, recent immune response research efforts have employed the antibody titers of those 2 IgG isotypes as an index of Th1 and Th2 cytokine activity. In the present study, we evaluated the antibody titers of those 2 isotypes, IgG1 and IgG2a, to confirm the Th responses. Similar to the results of cytokine mRNA expression, which was unbiased toward either Th1 or Th2 cytokine in the present study, similar expression levels of the 2 isotypes were also observed. However, the expressional condition of IgG1 was similar to that of IFN-γ and that of IgG2a was fairly similar to that of IL-4. The mRNA expression reached maximal levels during weeks 1-2 PI. Although mRNA expression achieved maximal levels on weeks 1-2 PI, the antibody titers increased rapidly during week 4. This could be explained by the assumption that there may exist some intermediate stages between mRNA expression and antibody formation controlled through several mechanisms in stages. Thus, the period of mRNA expression and antibody production may not necessarily correspond.
In the present study, we observed that the cytokine expression was unbiased toward either the Th1 or Th2 in immune response by times during infection, that the cytokine expression was controlled by the spleen rather than by the MLN, and that the cytokines assessed in this study were expressed at high levels during the early stages of infection (1-2 weeks). In addition, the IL-5 cytokine was suggested to respond to the worm activity, and IFN-γ was associated to egg deposition.