Serodiagnosis of Extraintestinal Amebiasis: Retrospective Evaluation of the Diagnostic Performance of the Bordier® ELISA Kit
Article information
Abstract
Soluble antigens from an axenic culture of Entamoeba histolytica were used to develop a commercial ELISA kit to quantify anti-E. histolytica antibodies in sera of patients with extraintestinal amebiasis in non-endemic settings. The diagnostic specificity and sensitivity of the test were assessed retrospectively using 131 human serum samples with amoebic serologic status available. They were selected according to their results in immunofluorescence (IFAT) and were separated in 2 sample categories: 64 sera with positive results by IFAT and 67 with negative results by IFAT. The sensitivity and specificity of the ELISA kit were assessed at 95.0% and 94.0% compared to the IFAT. The test can be useful to exclude a potential diagnosis of amebiasis and could be used as a screening method since ELISA is an automated technique.
In industrialized countries, amebiasis occurs in immigrants, tourists having travelled to endemic areas, sexually active homosexual men, and HIV positive individuals [1]. The primary target organ colonized by the parasite is the intestinal sigmoid and colon mucosa, where only highly invasive strains of Entamoeba histolytica species can display their invasive potential by producing tissue damage [2]. The most frequent form of extraintestinal amebiasis is the amoebic liver abscess (ALA) [3]. However, diagnosing ALA may be difficult due to the technical difficulties associated with distinguishing amoebic from pyogenic abscesses using ultrasound or radiological examinations [4]. The definitive diagnosis of ALA is based on the positive serological detection of anti E. histolytica antibodies in non-endemic settings [5].
This retrospective study evaluated diagnostic performances of the E. histolytica IgG ELISA kit provided by the Company Bordier Affinity Products (Crissier, Switzerland) for the serodiagnosis of extraintestinal amebiasis compared to IFAT.
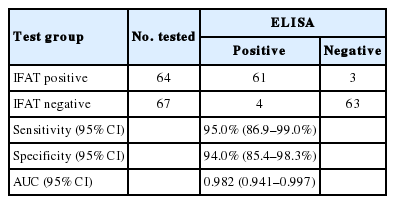
Serum samples were collected retrospectively from the officially approved biobank of the Grenoble Teaching Hospital Parasitology-Mycology laboratory. Selection criteria were the request for amoebic serology by clinicians and availability of IFAT results. Total 131 serum samples were selected and separated into 2 categories according to the retrospective biological record review of their amoebic serology results: 64 sera with positive results by IFAT (the routinely used commercial kit: Amoeba-Spot IF bioMerieux®) and 67 with negative results by IFAT. A serum titer>100 was considered as positive according to the manufacturer’s instructions. No other methods (PCR, microscopy, histology, or antigen test on stool or pus) were performed to exclude or confirm ALA.
The manufacturer provided prototypes kits designed as follows: the antigen was a crude extract from E. histolytica trophozoites of HM-1: IMSS strain, grown axenically at 37°C in sterile medium TYI-S-33 supplemented with 10% fetal bovine serum. Trophozoites were harvested by centrifugation. The parasites were frozen with dry ice and then thawed in the presence of proteinase inhibitors E64 (0.1 mM), the process was repeated 5 times. The suspension was centrifuged at 5,000 g and the supernatant was stored at −20°C.
The Maxisorp microplate wells (Nunc, Roskilde, Denmark) were precoated with 100 μl of amoebic antigen at a concentration of 5 μg/ml in carbonate buffer 0.1 M, pH 9.8. The plates were kept at 4°C overnight. The plates were dried at room temperature for 3 hr after washing with tris-buffered saline (TBS) containing 0.05% of Tween 20 (TBS-T). Strips of 8 breakable wells were stored in resealable aluminum foil containing desiccant and kept at 4°C.
Assays were performed according to the following manufacturer’s instruction: all serum samples were stored at −20°C until tested in dilution 1:200 TBS-T. 100 μl of diluted patient serum or positive, cut off and negative rabbit serums (supplied with the kit) were added to each well. The plate was then incubated at 37°C for 30 min and the wells washed 4 times; and 100 μl of conjugate protein-A labeled with alkaline phosphatase was added. The plate was incubated for another 30 min at 37°C and washed 4 times to remove unbound enzyme before adding 100 μl of freshly prepared substrate (para-nitro-phenyl phosphate) at a 2 mg/ml concentration. After 30 min at 37°C, the reaction was stopped by adding 100 μl of potassium phosphate pH 13.5. The optical density (OD) was measured at 405 nm in a ELx808 absorbance reader (Biotek, Winooski, Vermont, USA). The test is considered negative when the absorbance of the analyzed sample was lower than the absorbance of the weak positive serum; it was positive when the absorbance was higher than the absorbance of the weak positive serum. The weak positive serum (cut off) was manually determined by the manufacturer according to the results of a previous study on 20 serum samples of patients presenting positive amoebic serological results (Bern University) and 100 serum samples of blood donors (blood transfusion center, Geneva, Switzerland). No other methods (PCR, microscopy, histology, or antigen test on stool or pus) were performed to exclude or confirm ALA.
The test parameters were determined using 2×2 contingency tables: sensitivity, specificity. These values were compared using a student test. A P-value<0.05 was considered to indicate a significant difference. Area under the receiver operating characteristic curve (ROC-AUC) was calculated with MedCalc 11.6.0.0 (MedCalc Software bvba, Ostend, Belgium).
The clinical data of 64 patients with positive IFAT result was: ratio of males to females of 5.5, a median age of 40 years (range 7–71) correlating with the data reported by [6]. 89% patients presented with a liver abscess and 44% of these were punctured. The microscopic examination of the fluid never revealed ameba. 87% of the patients presented with abdominal pain, 84% with fever, 50% with diarrhea, and 16% with hepatomegaly in correlating with the data reported by [7]. Elevated C-reactive protein levels were observed in 80% of cases, and leukocytosis in 60% of cases. Hepatic cytolysis markers were elevated in 51% of cases. The same biological data was reported by [8]. This series of patients was representative of the population presenting with extra-intestinal amebiasis.
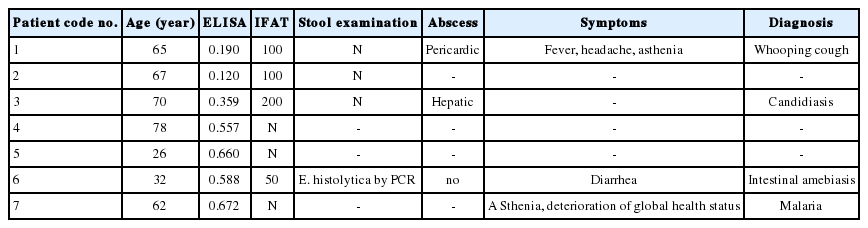
E. histolytica IgG ELISA had a sensitivity of 95% (61/64) and a specificity of 94% (63/67) compared to IFAT (Table 1). Student’s test showed no significant difference between ELISA and IFAT (P =0.9). ROC-AUC analysis showed AUC>0.9 (Table 1). ELISA OD values of negative IFAT samples (serum titer between 0 and 50) are spread between 0.030 and 0.672 (cut-off 0.409). ELISA OD values of positive IFAT samples (serum titer between 100 and 1,600) are spread between 0.120 and 3.513 (cut-off 0.409). The selection was made on the sole criterion of IFAT results and the clinical data of the 7 discordant cases was detailed (Table 2). Three patients (n° 1 to 3) had positive IFAT results and negative ELISA results. Four patients (n° 4 to 7) had negative IFAT results and positive ELISA results. The final diagnosis was not available for 3 patients (n° 2, 4, and 5) because the serum had been sent to the hospital for testing from external patient laboratories. There was no clinical diagnosis of amebiasis for 3 other patients (n° 1, 3 and 7). Patients 1 and 3 presented respectively whooping cough and candidiasis, 2 diseases which are not known to be responsible for cross-reaction with amebiasis serology. Patient 7 presented malaria, another protozoan disease which could be responsible for false positive results. Among the 131 samples, we found 6 patients with clinical diagnosis of malaria, and only one had positive results. The clinical data of patient number 6 showed that he presented with intestinal amebiasis, suggesting that the utility of the E. histolytica IgG ELISA for the diagnosis of intestinal amebiasis should be carefully analyzed.
As extraintestinal amebiasis presents with non-specific symptoms and direct detection is not always possible (except where PCR is available), the diagnosis is often based on epidemiological, radio-clinical, and biological data. The detection of serum specific antibodies is an important tool since microscopy is not sensitive and reliable enough [1]. E. histolytica antibodies are reliable markers for the diagnosis of extraintestinal amebiasis in non-endemic settings. There is a strong correlation between a high antibody serum titer and extraintestinal amebiasis [9]. The sensitivity and specificity of ELISA for the serodiagnosis of extraintestinal amebiasis was reported to range from 80% to 100% [10–14].
The diagnostic sensitivity and specificity of the E. histolytica IgG ELISA test were 95.0% and 94.0%, respectively, in our study. A sensitivity>94.0% and specificity>95.0% of serological tests were recommended [1], thus the E. histolytica IgG ELISA seems to be a suitable tool for the diagnosis of extraintestinal amebiasis among the wide range of available ELISA tests.
We showed that there was no statistical difference between the E. histolytica IgG ELISA and IFAT (P =0.9), as reported in other previous studies [15,16]. However, the analysis of discordant cases showed that some patients could be considered as positive with one technique and negative with the other. This stresses the importance to associate 2 complementary serological techniques, as recommended by experts [5]. Following this recommendation, the manufacturer of the E. histolytica IgG ELISA designed the cut off to have sensitivity as close as possible to 100%. The intention of use of the kit is screening of extraintestinal amebiasis as we know that there are other confirmatory assays available [1,7,15]. In this study, we have confirmed that the high sensitivity of the E. histolytica IgG ELISA can be useful to exclude a potential diagnosis of amebiasis and could be used as a screening method since ELISA is an automated method.
Notes
N. Beyls is employed by the company Bordier Affinity Products SA.