Comparative Characterization of Four Calcium-Binding EF Hand Proteins from Opisthorchis viverrini
Article information
Abstract
Four isoforms of calcium binding proteins containing 2 EF hand motifs and a dynein light chain-like domain in the human liver fluke Opisthorchis viverrini, namely OvCaBP1, 2, 3, and 4, were characterized. They had molecular weights of 22.7, 21.6, 23.7, and 22.5 kDa, respectively and showed 37.2–42.1% sequence identity to CaBP22.8 of O. viverrini. All were detected in 2- and 4-week-old immature and mature parasites. Additionally, OvCaBP4 was found in newly excysted juveniles. Polyclonal antibodies against each isoform were generated to detect the native proteins in parasite extracts by Western blot analysis. All OvCaBPs were detected in soluble and insoluble crude worm extracts and in the excretory-secretory product, at approximate sizes of 21–23 kDa. The ion-binding properties of the proteins were analyzed by mobility shift assays with the divalent cations Ca2+, Mg2+, Zn2+, and Cu2+. All OvCaBPs showed mobility shifts with Ca2+ and Zn2+. OvCaBP1 showed also positive results with Mg2+ and Cu2+. As tegumental proteins, OvCaBP1, 2, and 3 are interesting drug targets for the treatment of opisthorchiasis.
Opisthorchis viverrini is an important human parasite and chronic infection may lead to cholangiocarcinoma (CCA) [1]. The highest prevalence of the parasite infection is in the Lower Mekong basin, including Thailand, Laos, and Vietnam [2,3]. Praziquantel is the first choice of drug for opisthorchiasis and other foodborne trematodiases. Praziquantel-resistance has been reported in Schistosoma mansoni and possibly in S. japonicum [4], but as yet there has been no report of it in O. viverrini [5]. The mechanism of praziquantel against helminths is still unclear [6], but it involves tegumental damage caused by vacuolization rupture [7,8]. Tegumental antigens have been intensively studied for the development of drug targets and diagnostic tools. Calcium binding proteins (CaBPs) comprising 2 EF hand motifs and a dynein light chain (DLC) like domain have been identified and characterized in trematodes including Fasciola sp., Schistosoma sp., Clonorchis sinensis, and O. viverrini. Many of these CaBPs are located at the tegument layer of the parasites. The schistosome CaBPs, termed tegumental allergen-like proteins (TALs) [9], and Fasciola gigantica CaBPs [10], can strongly induce an IgE immune response in the hosts. However, the cellular mechanisms in which this protein family is involved remain unclear. In vitro studies showed interaction of S. mansoni and F. hepatica CaBPs [11,12] with drugs including praziquantel suggesting that these proteins merit further investigations. In the following, we briefly describe a basic molecular analysis of 4 CaPBs from O. viverrini, which has been done in preparation for research on their suitability as drug targets.
All animal experiments in this study were approved by the Thammasat University Animal Ethics Committee (project no. 014/2557, 28 October 2014). Syrian golden hamsters (Mesocricetus auratus) were infected with O. viverrini metacercariae collected from naturally infected fish to obtain immature and mature parasites. Female ICR mice were used for immunization to generate anti-recombinant OvCaBP antisera. All experimental details can be found in the Supplementary Text and Supplementary Tables 1–3. The cDNAs encoding 4 O. viverrini calcium binding proteins, i.e., OvCaBP1, 2, 3, and 4 (numbers solely appended for discrimination), and calcium binding experimentally verified as described below (GenBank no. MF 767953-MF767956), were isolated by reverse transcriptase PCR (RT-PCR) from adult stage total RNA extracted in TRIzol using primer pairs (1) ggatccATGACACAACAAGCAGCACA, aagcttCTATGCGCGGTTAGTACG; (2) ggatccATGGAAGGCATTGAATCAATG, aagcttTTAACACTGAGGGGTGCG; (3) catatg-GCACAGGTTCAAACG, ctcgagCGTCCGGTTCGTACGCCA; (4) ggatccATGGGTGAACAAGGATCG, aagcttTTAGTTGATGGTGGTACG) and inserted into pGEM-T Easy. They were selected from 19 family members that were identified by BLAST searches in the genome/transcriptome data of O. viverrini and C. sinensis [13–16]. Analysis of the deduced amino acid sequences showed that the 4 OvCaBPs had 37.2–42.1% sequence identity with O. viverrini calcium binding protein OvCaBP22.8 which until now had been the only reported family member in this parasite [17]. OvCaBP22.8 was identified by immunoscreening with the serum of a CCA patient, interestingly it was not detected in the parasite tegument but in the digestive tract and parenchyma. Predicted proteins GAA34310, GAA47752, and GAA37705 in the draft genome of C. sinensis [4] and characterized tegumental protein CsTegu21.6 [18] showed very high sequence identity (91–98% by NCBIBLASTP) with OvCaBP1, 2, 3, and 4, respectively, and obviously represent the orthologs in this closely related species. The sequence of each OvCaBP contains a pair of EF hand motifs in the N-terminal half, and a DLC-like domain in the Cterminal half (Fig. 1), as predicted by PROSITE and InterPro. The deduced numbers of amino acids of OvCaBP1-4 are 195, 184, 201, and 200 residues, with molecular weights of 22.7, 21.6, 23.7, and 22.5 kDa as calculated in EMBOSS pepstats [19], respectively. In pairwise comparison OvCaBP1 and OvCaBP3 show the highest sequence identity at 52.2%, OvCaBP3 and OvCaBP4 the lowest at 32.6% (see Supplementary Tables and Figures for details, EMBOSS needle). Sequence conservation was in general higher in the calcium-binding regions of the EF hand motifs and the C-terminal half of the DLC-like domain (Fig. 1). While the orthologs in O. viverrini and C. sinensis were highly conserved at >90% identity, this was most often not the case when comparing paralogous family members and was evident by low bootstrap support values in the phylogenetic tree (Fig. 2; Supplementary Fig. 1) constructed in PhyML 3.0 [20]. OvCaBP1-3 represent previously uncharacterized CaBPs in both species.
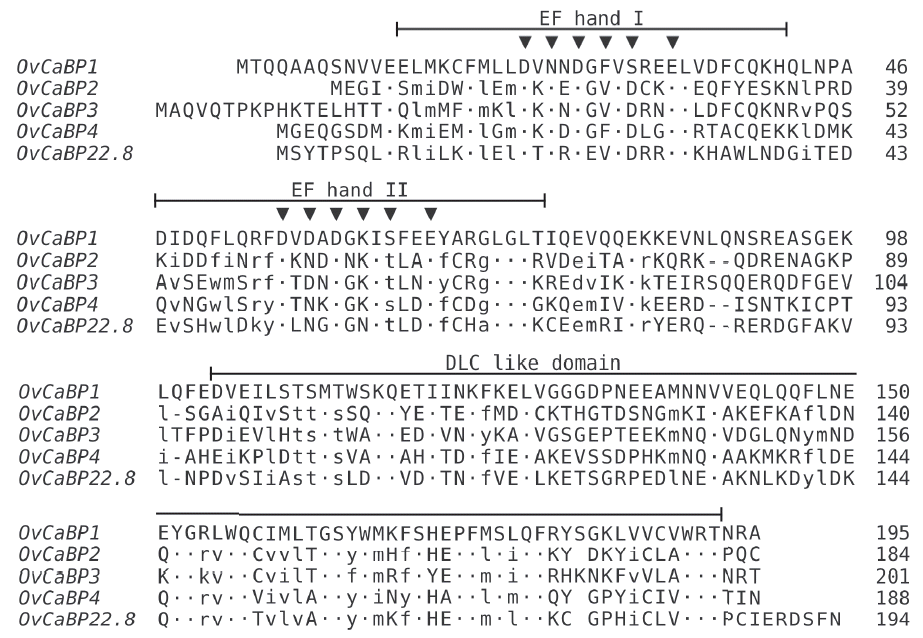
Multiple sequence alignment of OvCaBP1–4 and OvCaBP22.8. The 2 EF hand motifs and single dynein light chain like domain are indicated. The 6 residues in each EF hand motif making contact to calcium are indicated by triangles (▼). OvCaBP1 is used as reference sequence. Positions with all identical residues are indicated as dots ( · ), positions with all similar residues are shown in lower letters. Gaps introduced for alignment are indicated by dashes (-).
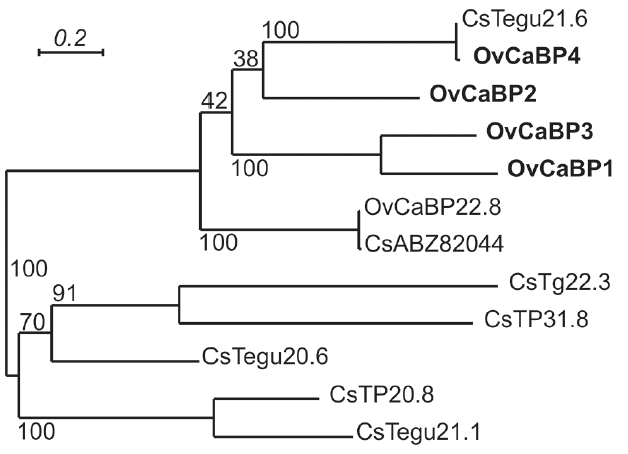
Phylogenetic tree based on maximum-likelihood analysis of characterized O. viverrini/C. sinensis CaBPs. The described OvCaBP1–4 are indicated in bold. CsTegu21.6, AEI69651, [18]; OvCaBP22.8, XP_009173200, [17]; CsABZ82044, ABZ82044, [27]; CsTg22.3, ABK60085, [28]; CsTP31.8, ABK60086, [29]; CsTegu20.6, GAA49981, [25]; CsTP20.8, ABC47326, [30]; CsTegu21.1, ADZ13689, [31]. The bootstrap support values are shown at the nodes, this is an unrooted tree, log likelihood: −3549.0.
Total RNA was extracted from newly excysted juveniles (NEJ) and 2-, 4-, and 8-week-old parasites by using TRIzol. The transcripts of OvCaBP1-4 were amplified by RT-PCR using the mentioned primers for each isoform and resolved by agarose gel electrophoresis. RNA products were found in 2-week-old juveniles through adult stage for all 4 genes. In addition, OvCaBP4 transcripts were faintly detected in NEJ (Fig. 3). Inferring from the results, the proteins are not important in dormant metacercariae and for excystation but are used in larger amounts in the parasite growth phase.
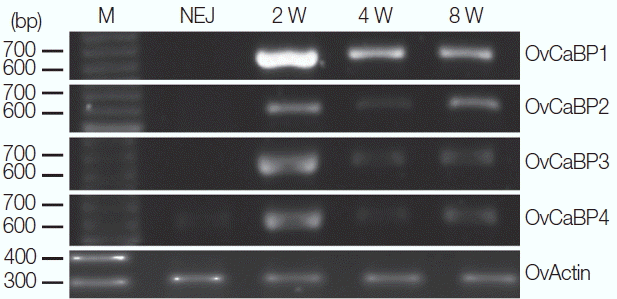
Stage-specific amplification of OvCaBPs transcripts by reverse transcriptase PCR. The total RNA of newly excysted juveniles (NEJ), 2-week juveniles (2 W), 4-week juveniles (4 W), and 8-week adult (8 W) O. viverrini were extracted in TRIzol and used as templates for RT-PCR with specific primers for each isoform. OvActin was used as standard. Lane M, 100 bp DNA ladder.
The OvCaBP cDNAs were subcloned into either prokaryotic expression vector pQE30 (N-terminal His-tag) or pET21b (Cterminal His-Tag, OvCaBP3) and recombinant OvCaBPs (rOvCaBPs) were expressed in soluble form in Escherichia coli and purified by metal affinity chromatography through the introduced histidine-tags (Supplementary Fig. 2). The metal ion-binding properties of rOvCaBPs were analyzed by mobility shift assays in non-denaturing PAGE (Fig. 4; Supplementary Fig. 3). Ca2+ and Zn2+ were bound by all rOvCaBPs. Mg2+ was bound by rOvCaBP1 and 3, Cu2+ only by rOvCaBP1. The metal ion binding properties of CsTegu21.6 [18], the ortholog of OvCaBP4 in C. sinensis were not reported. Binding with other divalent cations has been described for Sm20.6, Sm21.7, Sm20.8, FhCaBP2 and FhCaBP3 [11,12,21]. The binding of Ca2+ and other metal ions has been shown to take place at the helix-loop-helix structure of the EF hand motifs [22]. Binding with metal ions might have an effect on protein structure and therefore affect protein function.
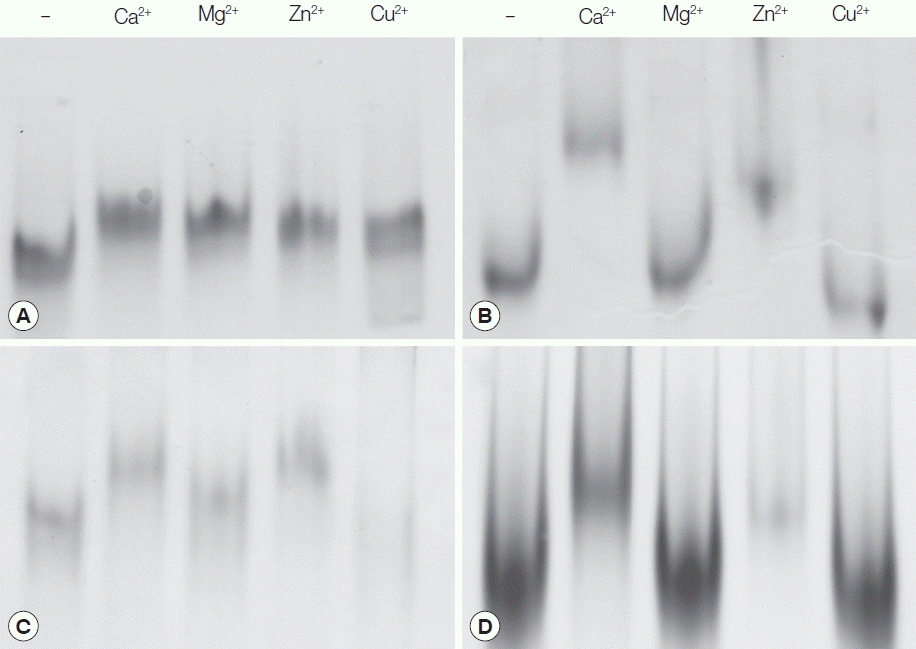
Determination of ion-binding properties by mobility shift assays in non-denaturing gels. Five micrograms of rOvCaBP (A-D) were pre-incubated with 5 mM EDTA and post-incubated with 25 mM CaCl2, MgCl2, ZnSO4, and CuSO4. Minus (−) symbols indicate proteins only incubated with 5 mM EDTA.
The purified rOvCaBPs were used to produce antisera in mice (3 mice per antigen) and these antisera were then used for western detection of the recombinant and native antigens. The antigens were resolved by SDS-PAGE and transferred onto nitrocellulose membranes by semidry blotting. The antisera detected recombinant and native antigens at their expected molecular weights in soluble and insoluble crude worm extracts (CW) and ES product (Fig. 5). Presence of OvCaBPs in the ES product suggests their release through non-classical secretion as they lack signal peptides. The antisera showed some cross-reactivity, potentially to other OvCaBP isoforms. Varying cross-reactivity was confirmed by testing each antiserum against the 4 rOvCaBPs in western blots (Supplementary Fig. 4) and has also been reported for CaBPs in F. gigantica [10]. This may be due to conserved immunodominant epitopes in their EF hand motifs as these could induce strong IgE immune responses in S. haematobium [23], S. mansoni [24], and F. gigantica [10]. OvCaBPs were detected immunohistochemically in the tegument and tegumental cell bodies of adult O. viverrini (Supplementary Fig. 5).
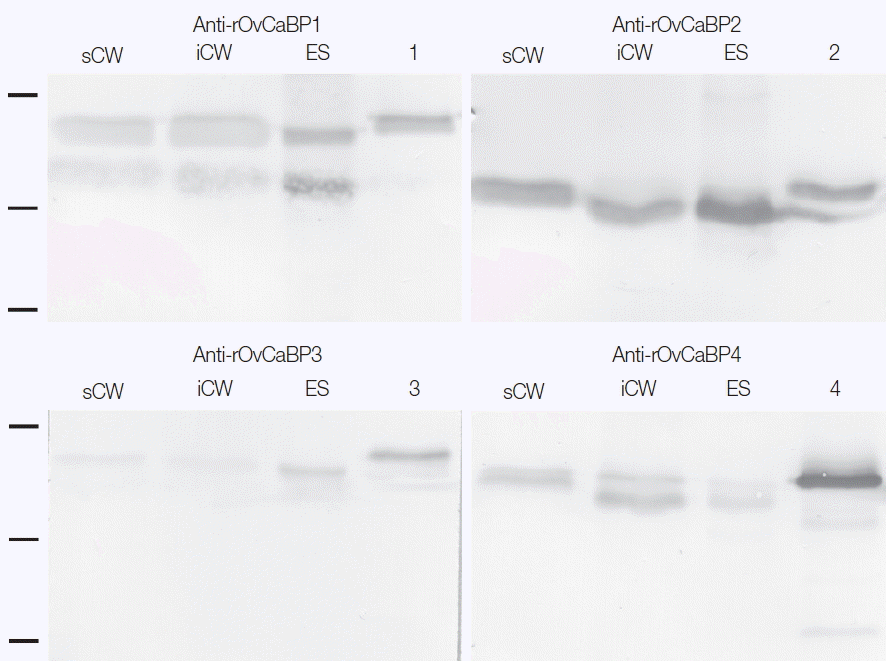
Western blot analysis of mature O. viverrini crude worm extracts (CW), ES product (ES), and recombinant OvCaBP1–4 (1, 2, 3, and 4) with mouse anti-rOvCaBP1–4 antisera at dilution 1:2,000. sCW, 20 μg soluble CW; iCW, 20 μg insoluble CW; ES, 20 μg ES product; lanes 1, 2, 3, and 4, 100 ng of rOvCaBP-1, -2, -3, and -4, respectively. Positions of 31.0, 21.5, and 14.4 kDa protein standards are indicated on the left.
In conclusion, the 4 described O. viverrini proteins are typical members of a large Platyhelminthes-specific subfamily in the family of calcium binding EF hand carrying proteins with the characteristic combination of 2 EF hand motifs and a single DLC like domain. The presence of all OvCaBPs in the ES product of the mature parasite was noted and preliminary data from infected hamsters suggests that at least OvCaBP2 is stimulating an immune response (data not shown). Despite modest sequence identity cross reactivity of antisera was observed which will impede application of OvCaBPs as diagnosis tools. On the other hand, the observed sequence divergences should result in isoform-specific surface structure/charge properties and future studies should focus on the drug binding potentials of the different isoforms.
Supplementary materials
Phylogenetic tree based on maximum likelihood analysis of 19 O. viverrini/C. sinensis CaBPs. The described OvCaBP1–4 are indicated in red color and previously analyzed C. sinensis and O. viverrini proteins in blue color (CsTegu21.6, AEI69651; OvCaBP22.8, XP_009173200; CsABZ82044, ABZ82044; CsTg22.3, ABK60085; CsTP31.8, ABK60086; CsTegu20.6, GAA49981; CsTP20.8, ABC47326; CsTegu21.1, ADZ13689). Uncharacterized proteins are named based on their accession numbers. The bootstrap support values are shown at the nodes, this is an unrooted tree, log likelihood: −7,368.24396
Purification of recombinant OvCaBPs under native conditions. Collected fractions were resolved by 12.5% SDS-PAGE including bacterial lysate (L), flow-through fraction (Ft), wash fractions (W1, W2) and elution fractions (E1–4). Each rOvCaBP was found highly purified at the expected mass in E1 and E2 fractions. Lane M: broad range protein standard marker (Bio-Rad).
Determination of calcium-binding property by gel mobility shift assay (8.5 or 12.5% non-denaturing gel). Five micrograms of the recombinant protein was pre-incubated with 5 mM EDTA and post-incubated with 25 mM CaCl while rSjGST was used as a negative control. Presence and absence of CaCl are indicated by plus (+) and minus (-) symbols, respectively.
Western analysis of cross-reactivity of mouse anti-rOvCaBP antisera. Each rOvCaBP was independently probed with each of the four anti-rOvCaBP antisera at a dilution of 1:2,000. Lanes 1–4: 100 ng of rOvCaBP1, rOvCaBP2, rOvCaBP3 and rOvCaBP4, respectively. Protein standard sizes are indicated on the left.
Immunohistochemical detection of OvCaBPs in adult O. viverrini tissue by mouse anti-rOvCaBP antisera at dilution 1:1,000. Positive red staining is present in tegument and tegumental cell bodies (OvCaBP1 and OvCaBP2) of the parasite (A, C, and D). No staining was observed in the tissue-sections detected with pre-immune sera (B and E). Ca, cecum; Eg, egg; Os, oral sucker; Pa, parenchyma; Sr, seminal receptacle; Sv, seminal vesicle; Tc, tegumental cell bodies; Te, Testis; Tg, tegument; Vs, ventral sucker.
ACKNOWLEDGMENT
Nucleotide sequence data reported in this paper are available in the EMBL, GenBank, and DDJB databases under the accession nos. MF767953, MF767954, MF767955, and MF 767956. This research was financially supported by a grant through Thammasat University, contract no: 35/2559 and 44/2560.
Notes
We declare that we have no conflict of interest related to this work.