Detection of Cryptosporidium spp. in Diarrheic Immunocompetent Patients in Beni-Suef, Egypt: Insight into Epidemiology and Diagnosis
Article information
Abstract
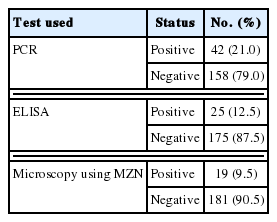
Cryptosporidium species is an important cause of gastrointestinal infections globally. This study aimed to shed light on its role in diarrheic immunocompetent patients in Beni-Suef, Egypt and to compare three diagnostic methods. Two hundred diarrheic patients, 37±16.8 year old, were enrolled. Stool samples were examined by light microscopy, using modified Ziehl-Neelsen stain (MZN) for Cryptosporidium spp. oocysts. Coproantigens were detected by sandwich ELISA. DNA molecular diagnosis was done by nested PCR. PCR yielded the highest detection rates (21.0%), compared to ELISA (12.5%) and MZN staining method (9.5%). The higher infection rates were in 20–40 year-old group, followed by 40–60 year-old. Association between epidemiologic factors was statistically not significant; positivity and gender, clinical manifestations, residence, source or water, or contact with animals. Cryptosporidiosis is an important enteric parasitic infection in Beni-Suef and PCR remains the gold standard for diagnosis.
INTRODUCTION
Cryptosporidium is a protozoan parasite involved in waterborne gastrointestinal infections. The disease usually is mild in immunocompetent patients [1,2]. Moreover, recent studies into the global causes of severe diarrhea in young children have identified the protozoan parasite Cryptosporidium as the second most important diarrheal pathogen after rotavirus [3,4].
The intake of unwholesome water could have devastating effects on our health, as unsafe drinking water is a key determinant of many microbial diseases with serious complications in immunocompetent and immunocompromised individuals [5,6]. Many pathogens are readily transmitted through drinking water including Cryptosporidium spp [7,8]. The presence of such parasites in drinking water is partly due to 4 reasons: Firstly, these organisms are indigenous pathogens found in most domestic animals; secondly, the degree of environmental contamination with their infective stages; thirdly, their resistance to water purification processes and finally unhygienic handling of drinking water [9,10]. Contrary to infection in the immunocompromised, cryptosporidiosis in otherwise healthy individuals is generally characterized by self-limited diarrhea, abdominal pain, weight loss, anorexia, fatigue, joint pain, vomiting and fever [10–12].
Laboratory diagnosis of cryptosporidiosis in stool samples depends on microscopic examination of stained slides, the most widely stain used is modified Ziehl-Neelsen (MZN), with a sensitivity of about 75% [13,14]. Other used methods include direct fluorescent antibody assay and coproantigen detection by immunochromatography [15–19]. High sensitivity and specificity for diagnosis was also reported with sandwich coproantigen detection by enzyme-linked immunosorbent assay (ELISA) [17,20] and molecular methods using polymerase chain reaction (PCR), targeting various genes, proteins and glycoproteins [21–24].
Few studies have been conducted in Beni-Suef, Egypt, on cryptosporidiosis in diarrhoeic apparently healthy patients [25]. Thus, this study aimed to document the occurrence of C. parvum among individual having diarrhea, at Beni-Suief University Hospital, using conventional copro-parasitological methods versus immunodiagnostic and molecular methods. It also aimed to develop guidelines for the investigation of diarrheic stool for the detection of C. parvum in such patients. Due to its high sensitivity and specificity, PCR has been put forward as the current gold standard [21,26–28]. Hence, it was chosen to be used in the current study for diagnosis of cryptosporidiosis, along with sandwich ELISA and microscopic examination of stool samples of the enrolled patients.
MATERIALS AND METHODS
Study population and ethical considerations
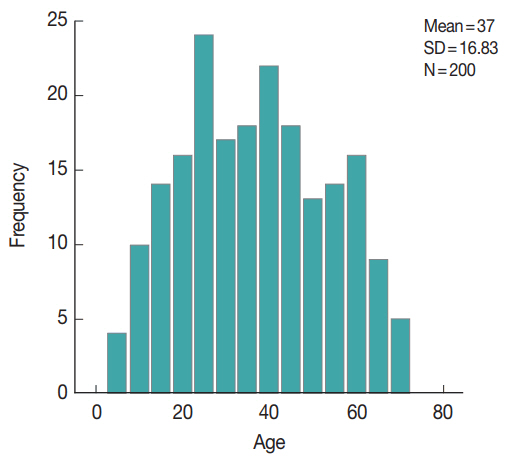
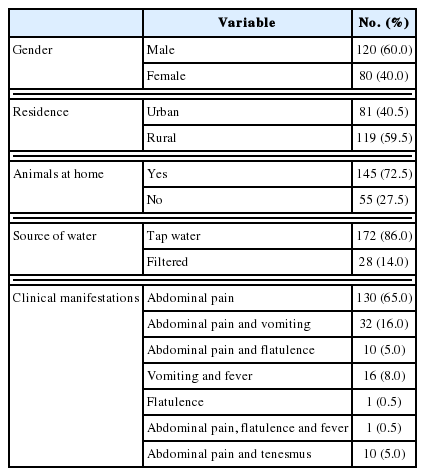
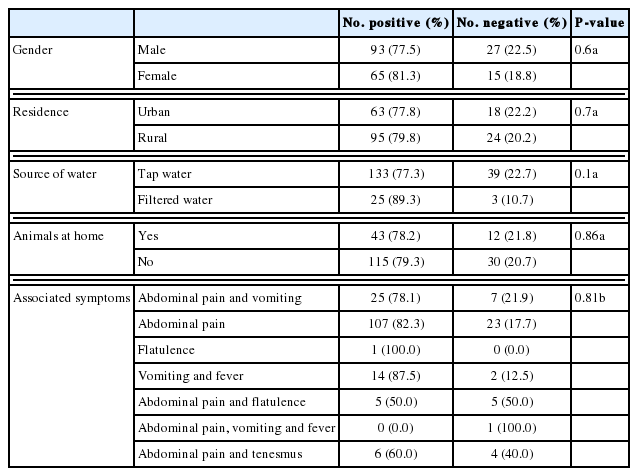
This was a cross-sectional study carried on stool samples from 200 diarrheic patients attending outpatient clinics of Beni-Suef University hospitals, from May 2015 to March 2016. The enrolled patients were from various age groups and both sexes (Fig. 1). Their demographic, history and clinical manifestations data are displayed in Tables 1, 2. The enrolled subjects were from various age groups with a mean age of 37 years (±16.8 SD). Males comprised 60% of the study subjects. The majority of the study patients was from rural areas, with access to tap water and reported the presence of domestic animals in proximity. The patients’ diarrhea had a mean duration of 3.8 days, with a mean of 8.5 for bowel movements. The main symptom reported was abdominal pain (194 patients, 97%), followed by vomiting (33 patients, 16.5%), fever (17 patients, 8.5%), flatulence (11 patients, 5.5%), and tenesmus (10 patients, 5%).
The study was approved by the ethical committee of Faculty of Medicine, Beni-Suef University and informed consent was obtained from patients or their relatives and parents of children. In addition, they responded to questionnaires related to history.
Sample collection and microscopy
A single fecal sample was obtained from each case. Then, Sheather’s sugar floatation concentration technique was done on part of the samples for microscopic examination. Also, acid-fast stained slides were examined for Cryptosporidium oocysts [29]. The remaining parts of the specimens were stored at −20°C for copro-immunoassays and molecular studies.
Coproantigen detecting ELISA
The frozen stool samples were subjected to Cryptosporidium coproantigen detection using sandwich ELISA (RIDASCREEN Cryptosporidium R-Biopharm Ag, Darmstadt, Germany) according to the manufacturer’s instructions. In brief, the test employs specific antibodies in a sandwich-type method. With the presence of Cryptosporidium antigens in a specimen, immobilized antibodies and the conjugated antibody form a sandwich complex. In order to establish a cut-off, 0.15 extinction units were added to the measured extinction of the negative control. Cut-off=extinction for the negative control+0.15. A specimen was considered positive if the extinction rate was more than 10% higher than the calculated cut-off value.
DNA extraction and nested PCR
Genomic DNA was extracted from the remaining frozen fecal samples using the FavorPrep Stool DNA Isolation Mini Kit (Favorgen Biotech, Ping-Tung, Taiwan) according to the manufacturer’s instructions. The samples were treated with thermal shock (5 cycles of deep freezing and boiling in a water bath, each for 5 min), incubated at 56°C for 10 min and prolonged for 1 hr at 95°C. Copro-DNA was extracted and amplified by nested PCR (nPCR) targeting COWP gene using two sets of primers: external primers, BCOWPF (5′-ACCGCTTCTCAACAACCATCTTGTCCTC-3′) and B C O W P R (5′-CGCACCTGTTCCCACTCAATGTAAACCC-3′), which amplified a 796-bp fragment [30]. A nested primer set cry-15 (5′-GTAGATAATGGAAGAGATTGTG-3′) and cry-9 (5′-GGACTGAA ATACAGGCATTATCTTG-3′) amplified a 553-bp fragment [31]. The amplified products were qualified by 1.5% agarose gel electrophoresis and ethidium bromide staining.
Statistical analysis
Data were analyzed using SPSS© Statistics version 24 (SPSS, Armonk, New York, USA). Skewed numerical data were presented as median and interquartile range and difference between groups was compared using the Mann-Whitney test (for 2-group comparison). Categorical variables were presented as number and percentage. Chi-square test, Fisher’s exact test, and Likelihood ratio tests were used to compare nominal data and the chi-squared test for trend to compare ordinal data. P-value was considered statistically significant if P <0.05. Receiver operating characteristics (ROC) with area under the curve analysis were used to confirm specificity and sensitivity of the diagnostic tests.
RESULTS
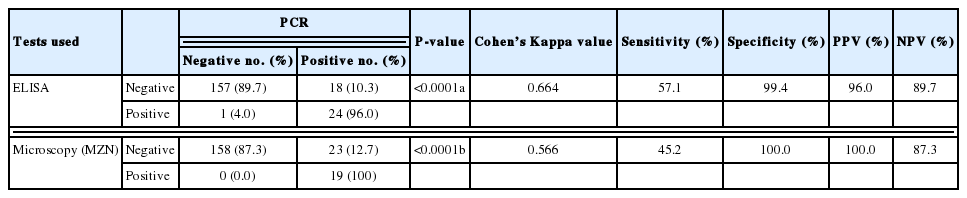
Table 2 shows the results of the examination of the stool samples by the 3 used methods in the study, with PCR as the gold standard (considered 100% sensitive and specific). On exploring the association between PCR and ELISA (Table 3), there was a statistically significant association between both test results (P <0.0001) as well as a statistically significant positive agreement between them (Cohen’s Kappa value 0.6–P-value< 0.0001).
On evaluating the diagnostic accuracy of ELISA in the current study, the test sensitivity was found to be 57.14%, test specificity was 99.3%, positive predictive value was 96% and negative predictive value was 89.7%. Microscopy with MZN staining had inferior sensitivity but 100% specificity and a comparable NPV to ELISA.
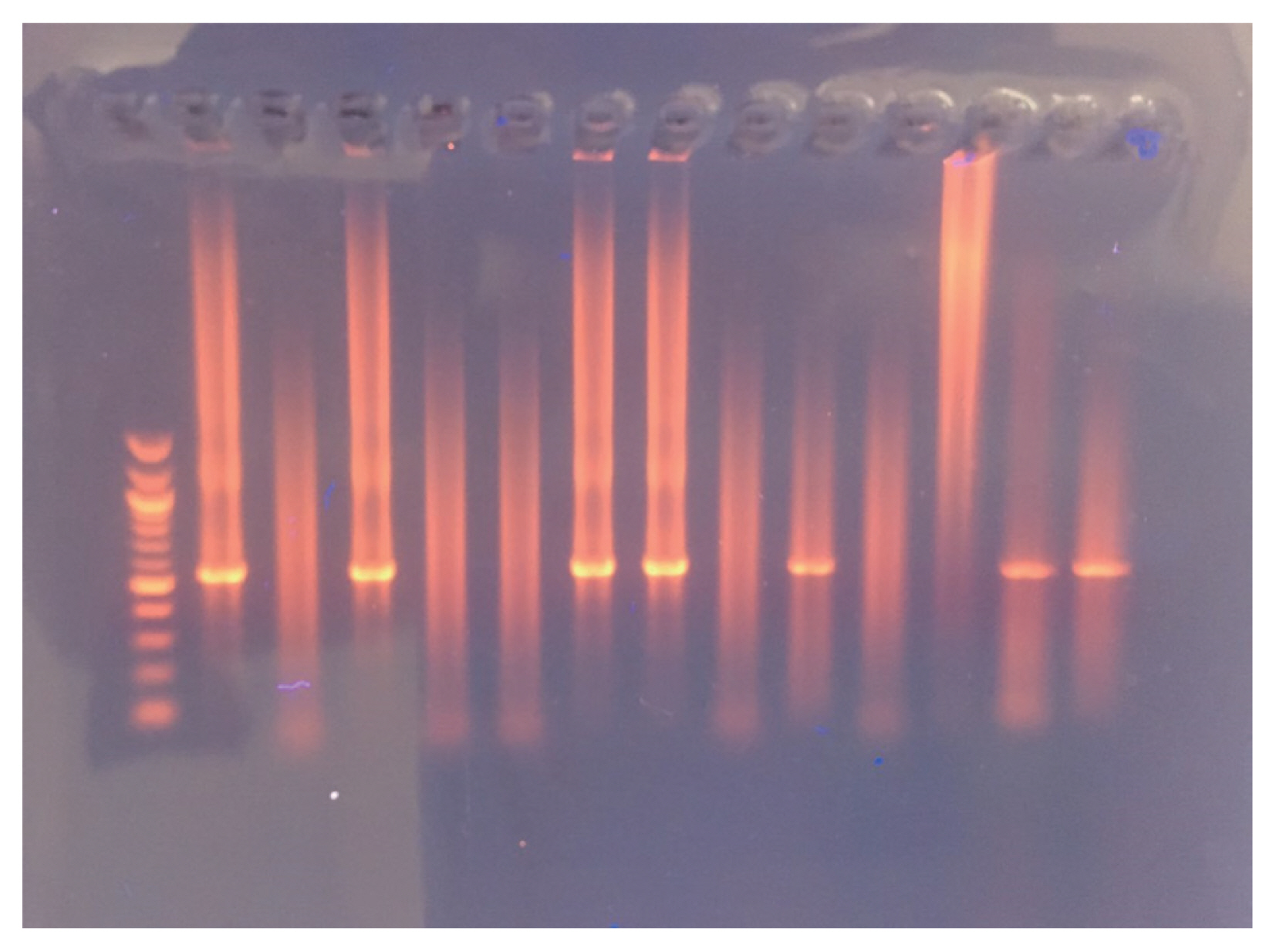
Fig. 2 depicts nested PCR results, negative and positive controls, as revealed by agarose gel electrophoresis. As for diagnosis by MZN stain, Cryptosporidium oocysts were found under microscopic observations (Fig. 3).
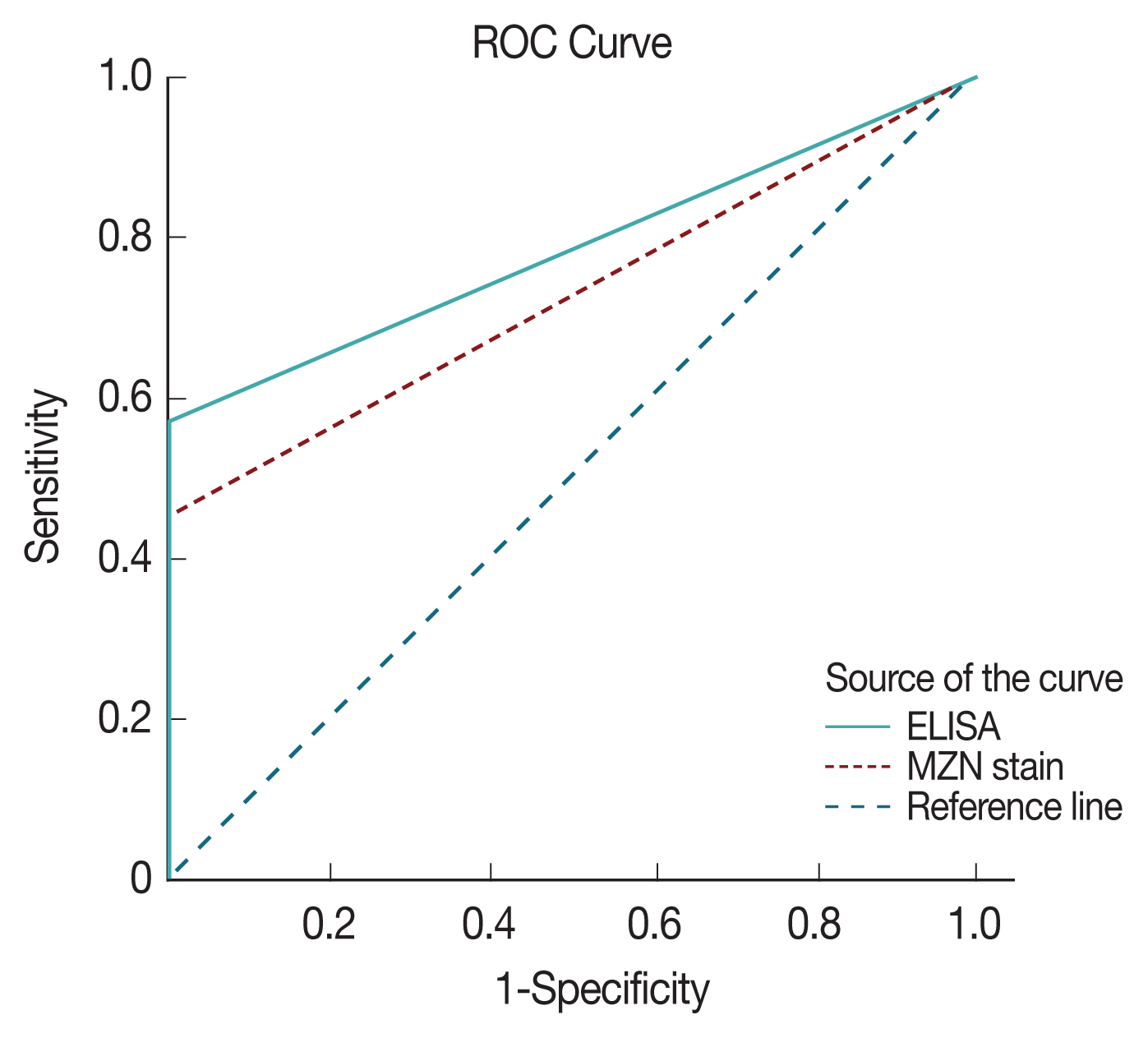
ROC with area under the curve analysis was used to test the specificity and sensitivity of both ELISA and direct microscopy in diagnosing cryptosporidiosis. Area under the curve value was 0.78 for ELISA and 0.73 for direct microscopy, respectively indicating both strong test specificity and sensitivity with a P-value of <0.0001 in both (Fig. 4). However, it is clear from results analysis that both have much inferior sensitivity compared to PCR results.
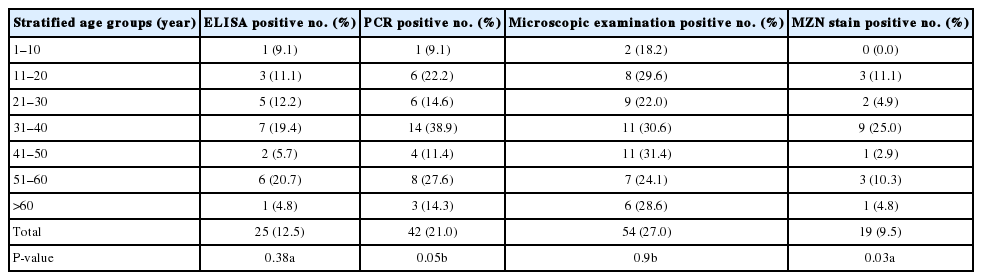
As seen in Tables 4 and 5, it was noted that adults aged 31–40 years had the highest infection rate, followed by those aged 51–60 years. Males were found to be more infected than females (22.5% and 18.8%, respectively). Tap water is found to be associated with higher infection rates, compared to filtered water (22.7% and 10.7%, respectively). Patients living in urban areas, with animal contact had higher infection rates, too.
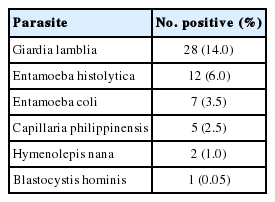
Other parasites were also discovered during the microscopic examination of the stool samples (Table 6). Ten of the cases infected with G. lamblia and 3 E. histolytica cases were also positive for Cryptosporidium.
DISCUSSION
Recently, PCR and immunodiagnostic methods have become more popular in diagnosis of cryptosporidiosis to overcome the limitations of microscopy. Compared with microscopy, DNA-based detection methods display several advantages such as an increased sensitivity and specificity, the possibility for molecular typing and an optimized turnaround time [27,32,33]. Given these trends in diagnosis, PCR was chosen the gold standard in this study. Indeed, it yielded the highest detection rates (21%), compared to ELISA (12.5%) and examination of MZN stained slides (9.5%) and this was found to be statistically significant. In accordance with previous works, findings of this study reaffirm the superiority of PCR in detection of Cryptosporidium in stool samples over immunodiagnosis and direct microscopy. PCR has a reported specificity of up to 100%, therefore, stool samples with oocyst-like bodies in MZN stained smears, but failed to yield specific PCR amplification are deemed negative. True, PCR nearly annuls chances of false positive results, however, this cannot be totally excluded [24,27,33,34]. Nevertheless, microscopy and ELISA are not likely to be excluded in field studies and laboratory lacking molecular diagnosis technology, especially in underdeveloped countries, due to their high specificity.
Beni-Suef is one of the less privileged governorates of Upper Egypt, with over 2 million inhabitants, about 80% of which live in rural areas [35], with substandard housing and drinking water condition, and most have domestic animals and pets in the vicinity. Infectious diseases, including cryptosporidiosis, are frequent there, being largely waterborne and zoonotic [19,25].
In Beni-Suef, an overall Cryptosporidium detection rate of 19% in human samples using MZN stains was previously reported [25]. Interestingly, the very same infection rate was obtained in the current study by using microscopy with MZN. Thus, it seems cryptosporidiosis in Beni-Suef has had a rather stable transmission patterns in recent years, even with an overall infection rate of 21%, according to PCR results.
Previously, it was stated that in humans, the highest prevalence of Cryptosporidium was in infants (31.3%) and diarrheic individuals (21.1%) [25]. As infants were not enrolled in this work and all patients were diarrheic, any comparison could not be made in this respect. Young adults were the most infected (31–40 years age group), followed by those aged 51 to 60 years. Needless to say, Infection rates by age and location differ around the world. For example, recently in a location in China the highest rate was found in 40–50 years age group, followed by elderly patients [36]. Environmental, hygiene and other epidemiological factors are involved in such variations. Cryptosporidiosis is classically regarded as particularly more common in children [19,37]. However, recent evidence suggests not to overlook it in adult and elderly diarrheic patients.
In this study, infection rates in males outnumbered females. Both similar and contradicting gender related cryptosporidiosis rates were previously reported; with females being more infected [38,39]. Variations in demographics and used diagnostic tests must be put in consideration.
In the current study, no statistically significant difference was found in infection rates comparing rural and urban residence, or the presence of animals at home. This may be explained by the fact that the routes of infection in this locality may be water borne, as 22.7% of positive cases consumed tap water, which is partially impure, compared to only 10.7% who reported basically drinking filtered water. Similar lack of statistical significance of the same factors was recently reported [40]. Contaminated food and both zoonotic and anthroponotic transmission cycles cannot be overlooked in an agricultural governorate like Beni-Suef, where urban areas may still be largely underdeveloped, especially in infrastructure, and where domestic animals, pets and stray animals are largely distributed.
The main manifestations of cryptosporidiosis, as reported in the study patients, were basically diarrhea, abdominal pain, vomiting and fever. None of the subjects was a documented immunocompromised case. A few other enteric parasites were detected in the study patients, and may have contributed to the symptoms of some. However, their presence does not seem to affect results of the current study, being of no statistical significance.
In conclusion, molecular diagnosis of human cryptosporidiosis is invariably superior to ELISA and direct microscopy after concentration and MZN staining, and remains the gold standard. Cryptosporidiosis remains an important cause of diarrhea in Beni-Suef, both in rural and urban areas, which share many environmental factors that favor transmission. Further studies into the transmission cycles and epidemiology of cryptosporidiosis in the Governorate, as well as characterization and genotyping of the parasites are needed for better management and control.
Notes
CONFLICT OF INTEREST
We have no conflict of interest related to this work.